Figure 1. TAK-242 oral gavage formulations and PK studies.
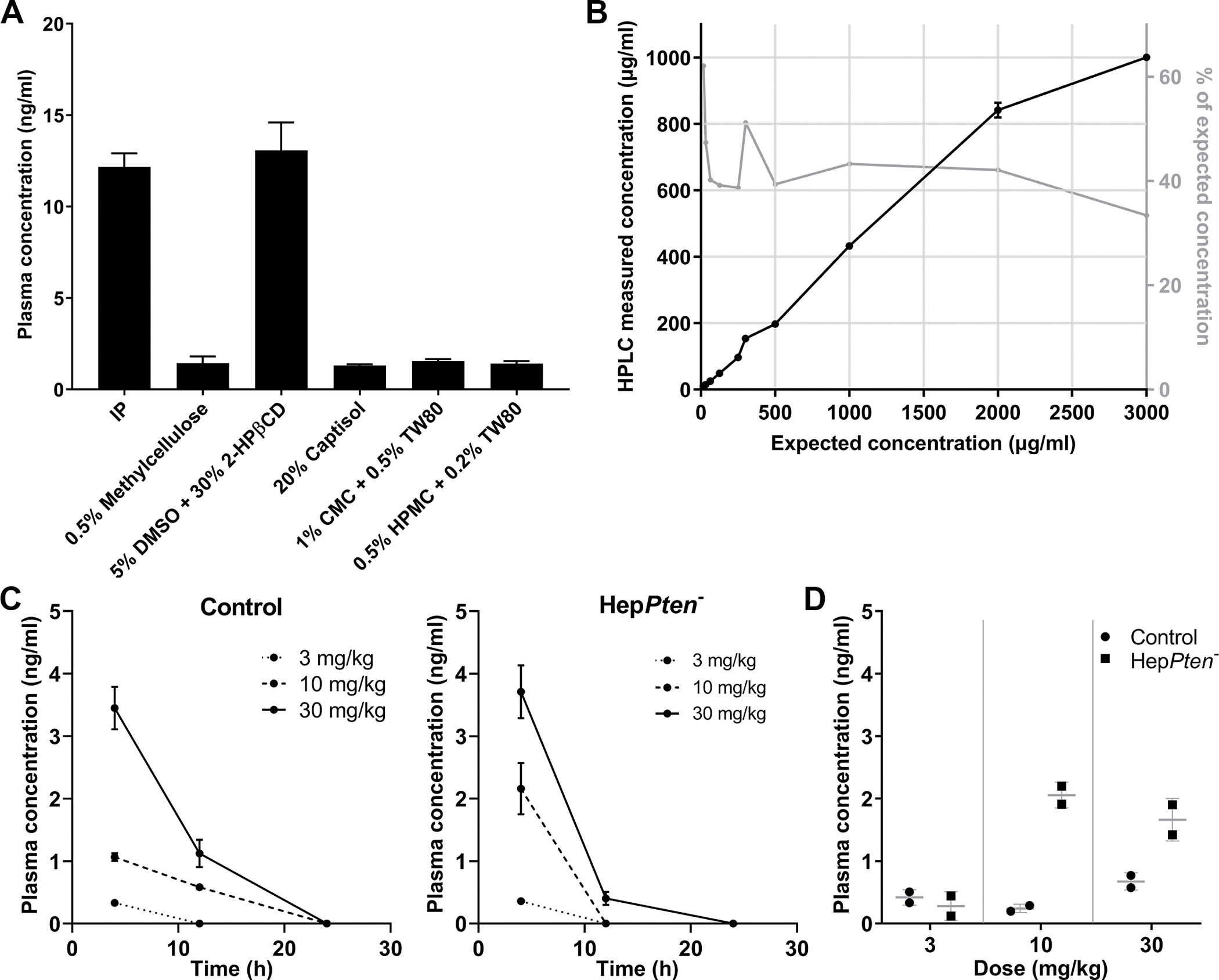
(A) Plasma concentrations of TAK-242 in mice 2 hrs after administration by IP or oral gavage of TAK-242 at a dose of 15 mg/kg. The oral formulations tested were: 0.5% methylcellulose, 5% dimethyl sulfoxide in 30% 2-hydroxypropyl-β-cyclodextrin (5% DMSO + 30% 2-HPβCD), 20% captisol. 1% carboxymethyl cellulose and 0.5% Tween-80 (1% CMC + 0.5% TW80), and 0.5% hydroxypropylmethylcellulose and 0.2% Tween-80 (0.5% HPMC + 0.2% TW80). (B) Solubility of TAK-242 in 5% DMSO + 30% 2-HPβCD assessed by HPLC. The concentrations tested ranged from 15 to 3000 μg/ml (equivalent to a mouse dosage of 0.15–30 mg/kg). (C) TAK-242 was administered by oral gavage as a single dose of either 3, 10 or 30 mg/kg to control and HepPten− mice and measured in plasma collected at 4, 12 and 24 hrs after administration. (D) TAK-242 was administered by oral gavage once daily for 5 days, at a dose of 3, 10 or 30 mg/kg to control and HepPten− mice and measured in plasma collected 2 hrs after last dose administration.
