Fig. 5. A-MYB/TCFL5 regulatory architecture establishes coherent feedforward loop to burst the expression of miR34/miR449 family, and a model for the transcriptional architecture of mouse male meiotic cells.
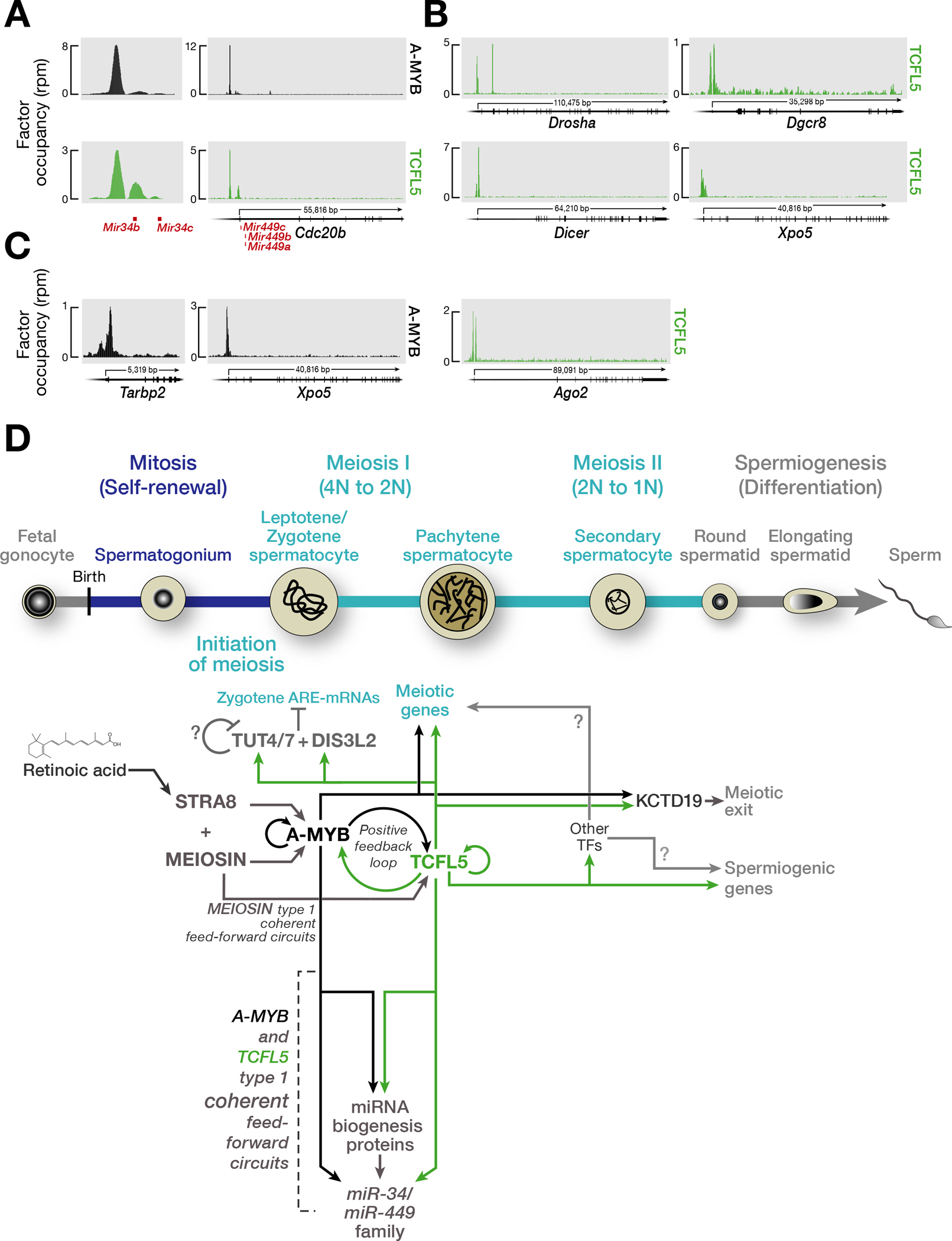
(A) A-MYB and TCFL5 CUT&RUN peaks at the promoters of miR-34b/c and miR-449a/b/c genes.
(B,C) A-MYB and TCFL5 occupancies around the promoters of genes encoding miRNA maturation proteins: TCFL5 occupancy (B) A-MYB occupancy (C).
(D) The model incorporates hypotheses from refs. (Bolcun-Filas et al., 2011; Galán-Martínez et al., 2022; Horisawa-Takada et al., 2021; Ishiguro et al., 2020; Kojima et al., 2019; Oura et al., 2021; Xu et al., 2022; Yu et al., 2022) and this study. The developmental progression of spermatogenesis is aligned with the time of expression of various proteins. The figure highlights the proposed sequential roles of STRA8, MEIOSIN, A-MYB, and TCFL5, calling attention to the underlying architecture of the transcriptional circuits regulating male meiosis in mouse.
