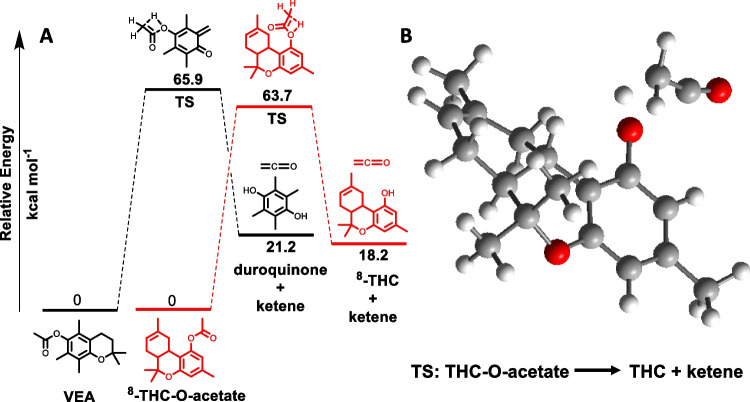
Fig. 2.
Calculated energy profiles for the pyrolysis of acetate substrates via concerted [1, 3] hydrogen shift mechanism using gas-phase M06-2X/6–311G (d,p) basis set. A Relative free energies (kcal mol−1) for starting materials, transition states, and products formed for the pyrolysis of VEA3 (black) and Δ8-THC-O-acetate (red) leading to the elimination of ketene. B Optimized geometry for the TS corresponding to the pyrolysis of Δ8-THC-O-acetate, leading to the elimination of ketene. Simplified structures used for THC calculations without C4H9 alkyl group.