Abstract
Chronic kidney disease (CKD) is a global health concern and public health priority. The condition often involves inflammation due to the accumulation of toxins and the reduced clearance of inflammatory cytokines, leading to gradual loss of kidney function. Because of the tremendous burden of CKD, finding effective treatment strategies against inflammation is crucial. Substantial evidence suggests an association between kidney disease and the inflammasome. As a well-known multiprotein signaling complex, the NLR family pyrin domain containing 3 (NLRP3) inflammasome plays an important role in inducing renal inflammation and fibrosis. Small molecule inhibitors targeting the NLRP3 inflammasome are potential agents for the treatment of CKD.The NLRP3 inflammasome activation amplifies the inflammation response, promoting pyroptotic cell death. Thus, it may contribute to the onset and progression of CKD, but the mechanism behind inflammasome activation in CKD remains obscure.In this review, we summarized recent findings on the role of the NLRP3 inflammasome in CKD and new strategies targeting the NLRP3 inflammasome.
Keywords: NOD-like receptor, Kidney function, Inflammasome, Chronic kidney disease
Abbreviations: CKD, Chronic kidney disease; ESRD, End-stage renal disease; NLRP3, NLR family pyrin domain containing 3; PAMPs, Pathogen-associated molecular patterns; DAMPs, damage-associated molecular patterns; ASC, apoptosis-associated speck-like protein; ROS, reactive oxygen species; LRR, leucine-rich repeat; TXNIP, thioredoxin-interacting protein; Ang II, Angiotensin II; HK-2, renal tubular epithelial cells; NF-kB, nuclear factor kappa-B; NEK7, NIMA-related kinase 7; IL-1β, Interleukin-1β; ,IL-18, Interleukin-18; GFR, glomerular filtration rate
Highlights
-
•
Chronic kidney disease (CKD) often involves inflammation due to the accumulation of toxins and the reduced clearance of inflammatory cytokines, leading to gradual loss of kidney function.
-
•
NLRP3 inflammasome plays an important role in inducing renal inflammation and fibrosis. Small molecule inhibitors targeting the NLRP3 inflammasome are potential agents for the treatment of CKD.
-
•
We summarized recent findings on the role of the NLRP3 inflammasome in CKD and new strategies targeting the NLRP3 inflammasome.
1. Introduction
Chronic kidney disease (CKD) is a common disease seriously endangering human health. The prevalence of CKD is rising with high public health costs and severe morbidity and mortality [[1], [2], [3]]. Classification of chronic kidney disease was shown in Table 1. In 2016, Global Burden of Disease Study ranked CKD as the 12th most common cause of death globally, affecting 13.4% of the global population [4]. According to an epidemiological survey, the global prevalence of CKD is 8%–16%, and the number of patients with the disease has reached 697 million, with about 132 million cases only in China [5,6]. Among patients over 80, more than 60% have CKD [7]. According to the United States Renal Data System 2019 Annual Report, the annual prevalence rate of CKD will reach 14.5%, and it is expected to increase by 16.7% in the United States by 2030 [8].
Table 1.
Classification of chronic kidney disease.
| Stage | Description | Glomerular filtration rate (GFR)(ml/min per 1.73 m [2]) |
|---|---|---|
| 1 | Kidney damage with normal GFR | >90 |
| 2 | Kidney damage with mild decreased GFR | 60–89 |
| 3 | Moderately decreased GFR | 30–59 |
| 4 | Severely decreased GFR | 15–20 |
| 5 | Kidney failure | <15(or dialysis) |
Renal fibrosis is a pathological process common to all renal diseases characterized by progression to end-stage kidney disease (ESRD). It is an international burden, affecting over 2 million people worldwide who require dialysis or renal transplantation.Low- and middle-income countries are the most vulnerable, as patients with end-stage kidney disease have difficulties accessing treatment [9,10]. Since CKD causes high lethality and is disabling, it has become a hot research topic in nephrology and concerning global public health problem [11].Because the pathogenic mechanism of CKD is unclear, only a few treatments for CKD exist [[12], [13], [14]], which provide symptomatic relief on patients with the disease.Multiple factors contribute to developing CKD: inflammation, apoptosis, oxidative stress, epithelial to mesenchymal transdifferentiation, extracellular matrix deposition, immunity, and many others. Inflammasome, especially NLRP3 Inflammasome, is of great important role in the occurrence and development of kidney diseases.
2. Structure and activation of NLRP3 inflammasome
2.1. Structure of NLRP3 inflammasome
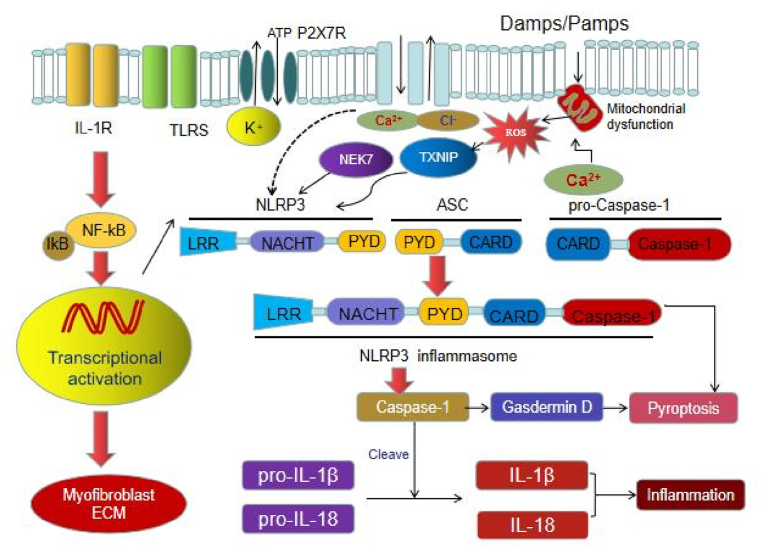
Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) are molecules that trigger innate immune responses. Damaged tissue, for example, releases DAMPs, causing a cascade of inflammatory mediators at the site of the injury. Both molecular patterns activate inflammasomes, multi-protein complexes composed of the PYHIN and Nod-like receptor family proteins, which play a vital role in the innate immune response [15]. Among them, NLRP3 is the best characterized inflammasome (Fig. 1). It mediates the inflammatory response against pathogenic microorganisms by activating caspase-1 and stimulating interleukin-1β (IL-1β) and interleukin-18 (IL-18) release to sustain homeostasis. The NLRP3 inflammasome consists of three proteins: NLRP3 scaffold, PYCARD (PYD and CARD domain containing) adaptor protein called apoptosis-associated speck-like protein (ASC), and caspase-1. NLRP3 protein is mainly composed of C-terminal leucine rich repeat (LRR), central nucleotide binding oligomerization domain (NACHT) and N-terminal pyrin domain (PYD). When exogenous microorganisms or endogenous tissue damage related molecules are recognized and combined by LRR, NACHT is oligomerized, and then PYD recruits ASC and pro-Caspase-1 to form NLRP3 inflammasome, pro-Caspase-1 is activated into Caspase-1 form, which induces the release of downstream IL-1β and IL-18 (Fig. 1) [16,17].
Fig. 1.
The NLRP3 inflammasome signaling transduction.
2.2. Activation of NLRP3 inflammasome
We know activation of NLRP3 inflammasome is a two-step process with an initiation and activation phase. During the initiation phase, TNF or Toll-like receptors activate nuclear factor kappa-B (NF-κB), upregulating NLRP3 and IL-1β proteins. In the activation phase, diverse DAMPs such as urate, cholesterol, and amyloid β-protein induce NLRP3 inflammasome assembly and subsequent activation. Because these factors do not directly interact with NLRP3, which is present in the intracellular fluid of immune cells, its activation is mediated by intermediate mechanisms, such as membrane damage and potassium ion efflux [18]. Upon activation, NLRP3 recruits the adaptor protein ASC through PYD–PYD interactions, polymerizing ASC. In turn, ASC recruits pro-caspase-1 that undergoes autocleavage into caspase-1, stimulating the maturation of IL-1β and IL-18 and triggering an immune response [19]. Because these factors do not directly interact with NLRP3, which is present in the intracellular fluid, its activation is mediated by intermediate mechanisms, such as membrane damage, increase in reactive oxygen species, lysosome disruption, and potassium ion efflux [20,21].
Recently, NLRP3 non-inflammatory functions have been discovered. For instance, in the renal tubular epithelial cells in a mouse model of unilateral ureteral obstruction, hypoxia may induce NLRP3 independently of ASC, IL-1β, and caspase-1 [22]. IL-1β is an important mediator of inflammation, and participates in a variety of cell activities, including cell proliferation, differentiation and apoptosis. IL-1β can destroy the structural integrity and function of podocytes by affecting the production of protein in podocytes. At the same time, it can destroy the glomerular filtration barrier by destroying the tight connection and adhesion of glomerular endothelial cells. IL-18, another important inflammatory cytokine in kidney, participates in and regulates the activation and differentiation of various T cells, and then participates in adaptive immunity [23]. IL-18 also mediates the production of other inflammatory cytokines, such as nitric oxide, cell adhesion molecules and chemokines, and induces the activation of inflammatory cells [24].In addition, Gasdermin D, as the downstream target of caspase-1, is induced by caspase-1 to crack and mature, thus playing an important role in cell scorch and IL-1β secretion [25] (Fig. 1).
3. Mechanisms of NLRP3 inflammasome activation
Many factors, such as high glucose, fatty polysaccharides and oxidative stress, can promote the assembly and activation of NLRP3 inflammasome [26,27](Fig. 2).In addition to NLRP3 inflammasome, NLRP3 protein also acts independently of inflammasome in the kidney.
Fig. 2.
The NLRP3 inflammasome activation pathways.
3.1. Ion signals
-
(1)
Potassium ion efflux. Potassium ion efflux is an essential mechanism that activates the NLRP3 inflammasome in macrophages. Low intracellular K+ levels activate the NLRP3 inflammasome and is sufficient for the activation [26]. A study demonstrated that a K+ efflux is an event upstream of NLRP3 inflammasome activation. Blocking inflammasome activation does not affect K+ efflux or macrophage activity. However, how K+ efflux orchestrates the NLRP3 inflammasome assembly is still unclear. Recent findings indicate that the decrease of intracellular K+ concentration leads to a conformational change in NLRP3, caused by NLRP3 mutation [27].
-
(2)
Calcium flux. Calcium-chelating agent BAPTA-AM inhibits the secretion of IL-1β after stimulation with ATP in macrophages, indirectly demonstrating that a Ca2+ signal is necessary for the NLRP3 inflammasome activation. Independent studies showed that calcium mobilization activates the NLRP3 inflammasome and raises bioactive IL-1β levels. Certain stimuli, such as alum and monosodium urate crystals, mobilize Ca2+ and activate the NLRP3 inflammasome. Moreover, they are sufficient to activate the inflammasome complex. mobilize Ca2+ and are sufficient to activate the NLRP3 inflammasome. Activated NLRP3 inflammasome causes Ca2+ mobilization, blocking Ca2+ signaling, and inhibiting the activation of NLRP3 inflammasome. Calcium flux does not affect other inflammasomes, suggesting that a Ca2+ signal is NLRP3 inflammasome specific [28,29]. Calcium phosphate crystals can also activate NLRP3 by destroying lysosomal membranes [30,31]. Although Ca2+ flux appears to activate the NLRP3 inflammasome and caspase-1, it occurs downstream of these components for some stimuli. Thus, whether Ca2+ flux affects the activation of NLRP3 inflammasome remains controversial.
3.2. Oxidative stress
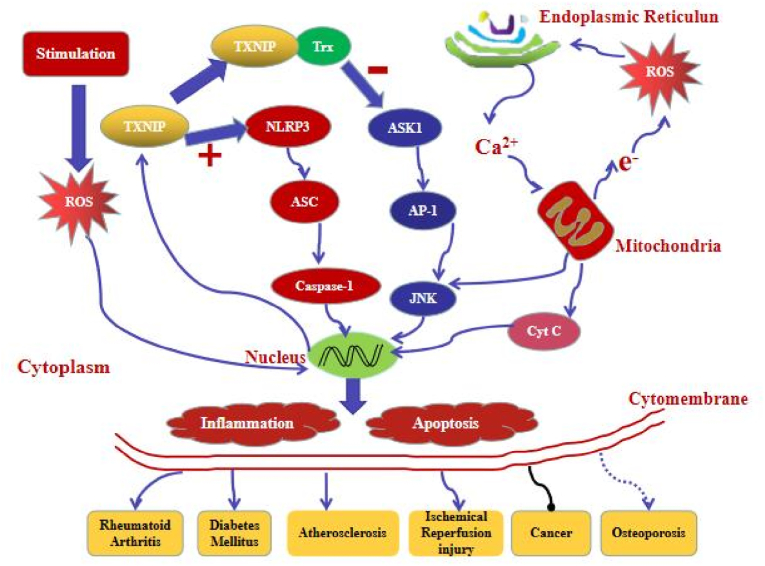
Oxidative stress is caused by a cellular rise in reactive oxygen species (ROS) and other free radicals. Some stress stimuli damage mitochondria or impair their function (e.g., NADPH oxidase), inducing ROS production and activating the NLRP3 inflammasome [32]. Watanabe et al. [33] found that endoplasmic reticulum stress enhances the secretion of IL-1β in human macrophages. Remarkably, IL-1β levels are proportional to ROS levels. We said that NLRP3 inflammasome activation were associated with ROS and potassium efflux. .Accumulation of misfolded protein in the endoplasmic reticulum promotes ROS and IL-1β production in human macrophages. The kidney is extremely sensitive to adversity, especially in critical developmental windows. During ischemic injury, the NLRP3 inflammasome damages kidney tissues [34]. The injury elevates mitochondrial ROS that induces thioredoxin-interacting protein (TXNIP), abundant in human glomeruli. The protein polymerizes with the NLRP3 inflammasome, activating the complex and mediating tissue damage. In vitro, it can increase NLRP3 expression, ROS production, caspase-1 activity, and apoptosis [35].It is also upregulated in conditions such as diabetes, rendering the kidney even more sensitive to ischemic injury.
3.3. Mitochondrial dysfunction
As introduced above, the NLRP3 inflammasome activity is closely associated with mitochondrial dysfunction. Mitochondrial ROS levels rise during cellular stress and are critical for the NLRP3 inflammasome activation [36]. Mitophagy, the removal of damaged or dysfunctional mitochondria, reduces ROS and inhibits the NLRP3 inflammasome. Excess ROS overstimulates the inflammasome, causing severe tubulointerstitial damage and apoptosis of renal tubular cells [37]. High urate levels stimulate the NLRP3 inflammasome activation by mitochondrial damage, contributing to ROS elevation [38]. The urate-activated NLRP3-ASC-caspase-1 axis locally triggers the inflammatory cascade, leading to hyperuricemic nephropathy and renal tubular injury [39].
3.4. Autophagy
Autophagy maintains intracellular homeostasis clearing away proteins, organelles, or intracellular pathogens [40]. There are three types of classical autophagy existing in mammalian cells [41].Macroscopically, two-membraned autophagosomes sequester cytoplasmic components such as damaged organelles or microbes. In microautophagy, lysosomes directly take up the components destined for degradation via membrane invagination. In chaperone-mediated autophagy, lysosomes selectively degrade cellular proteins, directly translocating them across the membrane. The selectively depends on the molecular chaperones that bind only proteins with specific recognition motifs. Autophagy is low under normal conditions. Conversely, it is high under stress conditions, such as hypoxia, DNA damage, and endoplasmic reticulum stress. Thus, increased autophagy regulates the functions of various organs, especially the metabolic [42].
The relationship between autophagy and kidney diseases has recently come under the spotlight. High glucose can induce oxidative stress in mesangial cells, resulting in aggregation of damaged mitochondria and ROS production, which may activate autophagy to clear damaged mitochondria and ensure energy recovery [43]. Excessive mitochondrial damage and oxidative stress induced by high glucose may also lead to excessive activation of autophagy and apoptosis [44].
3.5. Lysosomal disruption
Promoted by endocytosis of diverse particulates, lysosomal membrane degradation releases cathepsin B into the cytoplasm. Consequently, it activates the NLRP3 inflammasome [45]. Treating macrophages with CA-074-Me, a chemical inhibitor of cathepsin B, suppresses the inflammasome activation. Interestingly, the activation is absent when stimulating cathepsin B-deficient macrophages with particulates [46], indicating that inhibiting the inflammasome by the cathepsin B inhibitor may be due to a non-target effect or redundancy among cathepsin family members. In addition, siRNA- and shRNA-mediated knockdown of cathepsin B can promote the maturation of caspase-1 [47,48]. In some cases, cathepsin L can compensate for the missing cathepsin B, and its mode of action is similar to that of cathepsin B. Cathepsin C can supplement the activity of caspase-1; however, the function of cathepsin C requires further validation.
3.6. NIMA-related kinase 7
NIMA-related kinase 7 (NEK7), a multifunctional kinase, regulates mitotic spindle formation and cytokinesis, driving the cell cycle. Under normal growth conditions, it is a low-activity state. However, under pathological conditions, NEK7 switches to the high-activity state, producing numerous multinucleate and apoptosis cells and leading to an inflammatory reaction [49]. The activation of NLRP3 inflammasome is considered to be closely related to the regulatory factors of NEK7, as well as other signal events, such as K efflux and ROS. It is an essential modulator of the NLRP3 inflammasome that mediates its activation by binding to the leucine-rich repeat (LRR) domain of NLRP3 in a kinase-independent manner [50,51]. Since mitosis and activation of the NLRP3 inflammasome require NEK7, it may act as a switch between mitosis and inflammation to regulate their occurrence. Therefore, by acting as a switch between mitosis and the NLRP3-activated inflammatory response, NEK7 may prevent inflammatory damage during cell division [49,52,128].
The structural recognition between NLRP3 and NEK7 is confirmed by in vitro and intracellular mutations.The mutation of this interface removes the ability of NEK7 or NLRP3 to save NLRP3 activation in NEK7 knockout or NLRP3 knockout cells. These data suggest that NEK7 connects adjacent NLRP3 subunits through the interaction of two parts and mediates the activation of NLRP3 inflammasome [129].
Potassium efflux is a common step necessary for the activation of inflammasome in NLRP3 induced by various stimuli.NEK7 acts on the downstream of potassium efflux to regulate the oligomerization and activation of NLRP3.In the absence of NEK7, the activation of Caspase-1 and the beta release of IL-1 are cancelled in response to the signal of activating NLRP3, rather than the signal of NLRC4 or AIM2 inflammatory body.NLRP3 activation stimulates the interaction of NLRP3-NEK7, which depends on potassium efflux.NLRP3 is related to the catalytic domain of NEK7, but the catalytic activity of NEK7 is essential for the activation of NLRP3 inflammasome.Activated macrophages form a high-weight NLRP3-NEK7 complex, which is cancelled in the absence of NEK7 with the formation of ASC oligomerization and ASC spots.These studies suggest that NEK7 is an essential protein that acts downstream of potassium efflux and mediates the assembly and activation of NLRP3 inflammasome [50].
3.7. Thioredoxin-interacting protein
The thioredoxin (Trx) system is an important antioxidant system, which resizes oxidative stress by providing electrons to peroxides, thus enabling peroxides to effectively remove ROS and nitrogen. Thioredoxin-interacting protein (TXNIP) plays a vital role in cell death and immune response through interacting with the Trx system. TXNIP might be the key that links the hyperglycemic environment to inflammation by activating the NLRP3 inflammasome. As mentioned earlier, thioredoxin-interacting protein (TXNIP) is a critical activator of the NLRP3 inflammasome pathway. It plays an integral role in many diseases, including diabetic nephropathy and atherosclerosis. In normal circumstances, thioredoxin scavenges ROS, conferring tissue resistance to oxidative stress. However, TXNIP also directly binds thioredoxin, blocking its ROS-scavenging ability and limiting antioxidative defense.Therefore, a delicate balance exists between free and bound thioredoxin in tissues. When cells are exposed to a stress stimulus (e.g., high glucose), increased ROS production reacts with the TXNIP–TXN complex, dissociating it. Consequently, the released TXNIP activates the NLRP3 inflammasome, stimulating IL-1β and IL-18 maturation and downstream inflammatory events [[53], [54], [55], [56]].
A study [130] found that overproduction of mitochondrial reactive oxygen species (mtROS) is accompanied by decreased expression of TRX and up-regulation of TXNIP. In addition, the excessive production of mtROS in the kidneys of patients with diabetic nephropathy and db/db mice was also related to the increased expression of NLRP3/IL-1β and TGF-β.They reversed these changes by intraperitoneal injection of mitoquinolmesylate (MitoQ), an antioxidant of mtROS, into db/db mice. MitoQ can inhibit the dissociation of TRX and TXNIP, and then block the interaction between TXNIP and NLRP3, thus inhibiting the activation of NLRP3 inflammasome and the maturation of IL-1β in HK-2 cells.These results suggest that the activation of mitochondrial ROS-TXNIP/NLRP3/IL-1β axis is related to oxidative damage of renal tubules, and MitoQ can alleviate this damage by inhibiting the excessive production of mtROS.
Another study [131] confirmed that elevated plasma levels of s-adenosine homocysteine (SAH) in patients with diabetes are associated with renal dysfunction. It was found that adenosine dialdehyde (ADA) inhibition of S-adenosine homocysteine hydrolase (SAHH) can increase intracellular or plasma SAH levels, increase podocyte injury induced by high glucose, and aggravate STZ-induced diabetic nephropathy, which is related to the activation of NLRP3 inflammasome.Inhibition or knockout of NLRP3 can reduce podocyte injury and diabetic nephropathy aggravated by SAHH inhibition. In addition, SAHH inhibition increased TXNIP-mediated oxidative stress and activation of NLRP3 inflammasome, but these effects were not observed in TXNIP knockout mice.It is suggested that TXNIP/NLRP3 signal pathway is involved in diabetic nephropathy aggravated by SAHH inhibition.
3.8. EphA2
A study [132] found that EphA2, a member of the transmembrane tyrosine kinase receptor family, inhibits the activation of inflammasome in mouse airway epithelial cells (AECs) by tyrosine phosphorylation of NLRP3 in a reovirus infection model. In mechanism, EphA2 binds NLRP3 and induces its phosphorylation at Tyr132 site, which interferes with the formation of ASC spots and prevents the activation of NLRP3 inflammasome. As a negative regulator of NLRP3, EphA2 is considered to be a newly discovered phosphorylation site of NLRP3 and may be a potential therapeutic target for inhibiting NLRP3 inflammasome. At present, there has not been reported on whether EphA2 can inhibit NLRP3 inflammasome in CKD, and further studies are needed.
4. Chronic kidney disease and NLRP3 inflammasome
The clinical incidence of CKD is high, and patients often have poor prognosis. Immune dysfunction and inflammation, triggered by pathogens or immune cells, are independent risk factors for CKD. Because the NLRP3 inflammasome pathway is active in CKD, it has received much attention in nephrology. Thus, inhibiting or blocking the NLRP3 inflammasome activation cascade is one of the mainstays of therapy in renal diseases [57,58]. In the following sections, we will briefly summarize our understanding of how the NLRP3 inflammasome activation contributes to the pathogenesis of kidney-related disorders [59].The overview of role of NLRP3 in chronic kidney disease was showed in Table 2.
Table 2.
The role of NLRP3 in chronic kidney disease.
| Disease | Relevant factors change | Animal or cell | role | references |
|---|---|---|---|---|
| Hypertensive nephropathy | IL-1β↑,IL-18↑,ASC↑, Caspase-1↑,NLRP3↑ |
Mice,Glomerular podocyte | Sertoli cell apoptosis,Lysosomal membrane rupture,Renal fibrosis | 60–64 |
| lupus nephritis | NLRP3↑ | Glomerular podocyte | Proteinuria increase | 66 |
| SLE associated nephritis | ASC↑,Caspase-1↑, NLRP3↑ | Mice,Glomerular podocyte | Increase proteinuria | 64 |
| Mesangial proliferative kidney disease | ASC↑,Caspase-1↑, NLRP3↑ | Renal tubular epithelial cell | Renal interstitial inflammation,Renal tubular atrophy | 67 |
| Diabetic nephropathy | IL-1β↓, IL-18↓, ASC↓,Caspase-1↓ | NLRP3−/− mice | decrease inflammation in the kidney | 71–90 |
| Renal ischemia-reperfusion injury | NLRP3↑,ASC↑ | NLRP3−/− mice,Renal tubular epithelial cell | Decrease neutrophilinfiltration | 95–98 |
| Albumin induced nephropathy | NLRP3↑,Caspase-1↑,IL-1β↑,IL-18↑,Cathepsin B↑ | Mice,Renal tubular epithelial cell | Renal tubular cell apoptosis,Lysosome damage | 105 |
| Unilateral ureteral obstruction nephropathy | ASC↑,Caspase-1↑,IL-1β↑,NLRP3↑ | Mice | Renal tubular damage,renal interstitial fibrosis | 69,70 |
| High uric acid nephropathy | NLRP3↑,ASC↑ | Rat,Renal tubular epithelial cell | Renal epithelial-interstitial transformation | 101,103 |
| Azithromycin-treated nephropathy | NLRP3↑, lymphocyte↑ | Rat,Renal tubular epithelial cell | Lymphocyte infiltration | 102 |
4.1. The mechanism of NLRP3 inflammasome in hypertensive nephropathy
In mice with hypertensive nephropathy, the NLRP3 inflammasome inhibitor MCC950 binds NLRP3, alleviating inflammation and renal fibrosis [60]. Angiotensin Ⅱ (AngⅡ) plays a critical role in hypertensive nephropathy, and its infusion is used as a model of experimental hypertension in rodents. AngⅡ infusion model in mice suggests that AngⅡ treatment activates the NLRP3 inflammasome. Conversely, under conditions of NLRP3 deficiency, AngⅡ infusion-provoked mitochondrial dysfunction improves but does not affect hypertension [61,62]. Furthermore, AngⅡ stimulation can reduce the consumption of macrophages and increase the NLRP3 inflammasome formation [63]. AngⅡ infusion model in mice suggests that AngⅡ treatment activates the NLRP3 inflammasome because it is induced only in the treated mice [64].
4.2. The mechanism of NLRP3 inflammasome in chronic kidney injury
Inflammatory and immune responses contribute to CKD and could be its central mechanisms. Systemic lupus erythematous (SLE) mice gradually develop albuminuria, damaging renal tissues. Renal damage may be a consequence of the NLRP3 inflammasome activation [65]. In SLE patients with lupus nephritis, the increase in urine protein levels correlates with NLRP3 accumulation in glomerular podocytes [66]. This observation indicates that inflammasome activation is associated with SLE. Patients with mesangial proliferative glomerulonephritis exhibit a substantial accumulation of NLRP3 in renal tubular epithelial cells in addition to tubular atrophy [67]. This accumulation is also observable in the renal interstitium.The mRNA levels of NLRP3 in peripheral blood mononuclear cells of hemodialyzed CKD patients are higher than that of healthy subjects [68]. Hence, the inflammasome is activated in patients with uremia who received dialysis treatment. This non-physiological state might be caused by mitochondrial dysfunction, critical for the NLRP3 inflammasome activation.
4.3. The mechanism of NLRP3 inflammasome in UUO nephropathy
Unilateral ureteral obstruction (UUO), as a CKD model, provides a basis for the study of molecular and cellular mechanisms of renal fibrosis.NLRP3 inflammasome and its downstream cytokines increased in UUO mice [69].In addition, compared with the control mice, the renal fibrosis, ROS damage and apoptosis of UUO mice with NLRP3 gene knockout were weakened, and the mitochondrial morphology and function were less damaged [70].
4.4. The mechanism of NLRP3 inflammasome in diabetic nephropathy
Inflammation is a potential mechanism for the pathogenesis of diabetic nephropathy, which is associated with reduced kidney function and renal interstitial inflammation in diabetic patients [71]. Hyperglycemia activates NF-kB signaling in renal tubular epithelial, inducing pyroptosis and maturation of IL-1β and IL-18. Moreover, it is sufficient for the induction of downstream inflammatory events. As inflammation develops, TGF-β levels increase, promoting renal fibrosis [72]. Recent evidence shows that the NLRP3 inflammasome complex and its downstream factors IL-1β and IL-18 are substantially depleted in NLRP3 knockout mice, emphasizing that NLRP3 is necessary for inflammatory cytokine production [73]. The expression of IL-1β, NLRP3, and caspase-1 increases in rat mesangial cells after high glucose exposure, implying a time-dependent effect [[74], [75], [76]]. In patients with type 2 diabetes, the NLRP3 inflammasome complex is overactive, which normalizes following glibenclamide treatment. These findings suggest the NLRP3 inflammasome plays an important role in diabetes [[77], [78], [79], [80], [81]].
Glucose-induced inflammasome activation promotes transdifferentiation of renal tubular epithelial cells, indicating the NLRP3 inflammasome plays a key role in the transdifferentiation [82,83] Silencing NLRP3 and ASC, genes reduce levels of phosphorylated SMAD2 and SMAD3 in mouse renal tubular epithelial cells (HK-2) after TGF-β1 treatment, suggesting NLRP3 amplifies the TGF-β1 signal. The cumulative renal inflammatory factors are closely related to the deterioration of renal function in diabetic nephropathy [84]. For instance, the inflammatory response positively correlates with the expression of NLRP3, adrenergic receptor P2X4, and IL-18 in epithelial cells. Thus, adrenergic receptor P2X4 likely stimulates inflammatory cytokine production through the NLRP3 inflammasome activation. Consequently, the activation causes a interstitial inflammatory renal response in diabetic nephropathy. Activation of the NLRP3 inflammasome induces the production of proinflammatory factors and further promotes insulin resistance in DN patients [85]. In contrast, knockdown or inhibition of NLRP3 reduced diabetic kidney injury.
The study also found that the expression of NLRP3 inflammasome in the renal tubules of diabetic patients with tubulointerstitial injury was increased. Furthermore, NLRP3 inflammasome activation was observed in glomerular endothelial cells and podocytes of DN mice.The activation of NLRP3 inflammasome during DN development involves various pathways, such as the nuclear factor E2-related factor 2 (Nrf2) pathway [85], the ROS/TXNIP pathway [86], the NF-κB pathway [87], and the P2X7/NLRP3 pathway [88].In the DN rat model, mitophagy reduces the body inflammatory response and further damage by regulating the macrophage M1 and M2 ratio, and maintains the homeostatic in vivo [89]. A recent study [90] showed that NLRP3 mediates renal damage in a DN mouse model by inhibiting autophagy in podocytes.
4.5. The mechanism of NLRP3 inflammasome in IgA nephropathy
IgA nephropathy (IgAN) is one of the most common chronic kidney diseases. 25%–30% of IgAN patients develop kidney failure after 20 years of illness.NLRP3 inflammasome in macrophages of IgAN mice was activated by IgA immune complex, while renal damage in NLRP3 knockout mice was alleviated [91]. Dys-glycosylated IgA1 isolated from the serum of IgAN patients can induce NLRP3 expression in podocytes and induce podocyte macrophage transformation, which in turn leads to renal inflammation and fibrosis [92]. Abnormal deposition of glycosylated IgA1 induces the NLRP3 inflammasome activation and macrophage transdifferentiatio-n in IgA nephropathy [93]. Thus, this observation suggests one of its pathological mechanisms.These evidences show that NLRP3 plays an important role in the development of IgAN, and the specific mechanism needs to be further studied.
4.6. The mechanism of NLRP3 inflammasome in ischemia-reperfusion nephropathy
The kidney is an organ prone to ischemic injury, which can cause renal tubular damage [94]. Blood reperfusion is essential for the survival of tissues; however, it can also enhance the inflammatory response and aggravate renal tissue damage [95]. Expression levels of NLRP3, ASC, and other inflammasome-related genes significantly increase after renal ischemia-reperfusion injury in wild-type mice, causing neutrophil infiltration and renal damage [96]. Conversely, in NLRP3 knockout mice, pro-inflammatory cytokine levels and neutrophil infiltration significantly decrease after 24 h reperfusion, and the renal function substantially improves. In human and mouse in vitro experiments, NLRP3 and ASC are detectable in renal tubular epithelial cells [97].
After a renal ischemia-reperfusion injury in NLRP3 knockout mice, apoptosis and necrosis of renal tubular epithelial cells significantly decrease. However, when ASC and caspase-1 are knocked out in mice, renal function and tubular injury do not improve, suggesting that NLRP3 has independent roles in the inflammasome pathway. In NLRP3 knockout mice with leukocyte deficiency, apoptosis and necrosis of renal tubular epithelial cells decrease significantly [98]. Conversely, when leukocytes are present, repair of renal tubular epithelial cells increases. This experiment indicates that leukocyte-derived NLRP3 inflammasomes are related to apoptosis of renal tubular epithelial cells, while the renal-derived association with renal tubular epithelial repair after ischemia-reperfusion.
4.7. The mechanism of NLRP3 inflammasome in other kidney diseases
The expression of NLRP3 inflammatory complex increases in mice with tubulointerstitial injury, further exacerbating it. In unilateral ureteral obstruction mouse model, the NLRP3 inflammasome and its downstream factors IL-1β and IL-18 increase, driving the renal tubular damage and renal interstitial fibrosis [99]. During hypoxia in renal tubular epithelial cells, NLRP3 increases independently of caspase-1 and ASC, demonstrating its inflammation-independent function [100]. This finding agrees with a previous study that showed elevated NLRP3/ASC expression in renal epithelial cells of hyperuricemia rats and the renal epithelial–mesenchymal transition.
Allopurinol can reduce the activation of the NLRP3 inflammasome, significantly reducing renal fibrosis [101]. In azithromycin-treated nephropathy rats, azithromycin decreases IL-1β concentrations and NLRP3 mRNA transcription, suggesting it reduces inflammasome activation [102]. Renal tubular crystalline deposition can release irritants caused by leukocyte infiltration, inducing the NLRP3 inflammasome activation and the necrotic inflammatory response of the kidney [103,104]. Calcium oxalate crystals can directly damage renal tubular epithelial cells by releasing ATP to activate the NLRP3 inflammasome and cause renal tubular injury [105].
5. Drugs targeting NLRP3 inflammasome in kidney diseases
At present, there are many kinds of biological inhibitors against NLRP3 inflammasome (Table 3), but the efficacy and safety of these inhibitors for kidney diseases have not been defined.
Table 3.
The application of drugs for NLRP3 inflammasome in kidney diseases.
| Drug | Target spot | Mechanism | Kidney diseases | Adverse effect | Clinical stage |
|---|---|---|---|---|---|
| MCC950 [[106], [107], [108], [109], [110], [111]] | NLRP3 | NLRP3 induced ASC oligomerization was blocked | Diabetic nephropathy, Hypertensive nephropathy, Contrast medium nephropathy,Kidney damage caused by cisplatin and sepsis | Liver toxicity | Preclinical study |
| Tranilast [[112], [113], [114]] | NLRP3 | Enhanced NLRP3 ubiquitination,Binding NACHT and inhibiting NLRP3-NLRP3 interaction | Diabetic nephropathy | NA | Clinical application |
| Β-hydroxybuty -rate [115,116] |
NLRP3 | Inhibited K+ efflux and reduced ASC oligomerization and speckle formation | Hyperoxalate renal tubular injury | NA | Preclinical study |
| CY-09 [117,118] | NLRP3 | Bind to ATP binding motif of NACHT domain and inhibit NLRP3 ATP activity | Ischemia-reperfusion nephropathy | NA | Preclinical study |
| VX-740/765 [119] | Caspase-1 | Selective inhibition of caspase-1 | NA | Liver toxicity | Preclinical study |
| AZD9056 [120] | P2X7 | P2X7 Antagonistic P2X7 | NA | NA | Phase II clinical study |
| Brilliant blue G [121,122] | P2X7 | Selective antagonism of P2X7 | Hypertensive nephropathy, Lupus nephritis | NA | Preclinical study |
| Glibenclamide [[123], [124], [125]] | K+ channel | ATP sensitive K+ channel inhibitors | Chronic kidney disease | Abnormal glucose metabolism | Clinical application |
5.1. Drugs targeting NLRP3
MCC950, a diaryl sulfonylurea small molecule compound, is the most effective NLRP3 inhibitor with high specificity [106].MCC950 can reduce glomerular basement membrane thickening, podocyte injury and renal fibrosis by inhibiting NLRP3/caspase-1/IL-1β pathway in diabetic nephropathy model [107].MCC950 can reduce the blood pressure and urine protein level of hypertensive mice, and alleviate kidney inflammation and fibrosis.MCC950 can improve renal fibrosis by inhibiting the activation of inflammasome and the production of IL-1β and IL-18 in crystalline nephropathy [108].MCC950 can also reduce podocyte damage in obesity-related glomerulopathy, as well as kidney damage caused by sepsis [109].In addition, MCC950 can improve cisplatin-induced renal insufficiency by reducing oxidative stress and inflammation, improving renal tubular injury and renal fibrosis [110,111].Although the above research results have been obtained, and MCC950 has the advantages of small molecule and high specific-ity, the safety of its application in the treatment of kidney diseases remains to be clarified.
Tranilast, an analogue of tryptophan metabolites, is a traditional antiallergic drug. Recently, it has been confirmed that tranilast can directly inhibit NLRP3 activity [112].By enhancing the ubiquitination of NLRP3, combining with its NACHT domain and inhibiting the direct interaction of NLRP3-NLRP3, Nistrost interrupts the assembly and activation of NLRP3 inflammasome [113].Tranilast plays an important role in preventing the progression of renal fibrosis and can improve the nephrotoxicity induced by cyclophosphamide and cyclosporine [114]. Although tranilast has not been approved for the treatment of kidney diseases, the above research shows that it has shown great potential in animal experiments. It is expected to study its efficacy and safety in various kidney dis eases in the future.
β-hydroxybutyrate (BHB) is an inhibitor of NLRP3 inflammasome discovered in recent years, which can prevent K+ outflow and inhibit ASC oligomerization [115]. A research [116] found that BHB can alleviate renal tubular injury in mice fed with high oxalate diet, and can also change macrophage phenotype from pro-inflammatory phenotype to anti-inflammatory phenotype. It is suggested that BHB can alleviate kidney inflammation, and may become a potential drug for treating kidney-related diseases by inhibiting NLRP3 inflammasome.CY-09, a direct inhibitor of NLRP3 inflammasome, can inhibit the assembly and activation of NLRP3 inflammasome by binding to the ATP binding motif of NACHT domain and inhibiting the activity of NLRP 3 ATPase [117].CY-09 plays a role in animal models of various diseases. However, there are few reports on its application to kidney diseases. At present, only CY-09 has been found to improve renal insufficiency induced by I/R [118].
5.2. Drugs targeting caspase-1
VX-740 and VX-765 are peptidomimetic prodrugs that inhibit caspase-1. The clinical trials applied to psoriasis, arthritis and epilepsy have progressed to phase II, but they have been forced to stop because of hepatotoxicity [119]. Up to now, there is no relevant clinical trial about its application in kidney diseases.
5.3. Drugs targeting P2X7
AZD9056 is the first P2X7 receptor antagonist to pass clinical trials, which can significantly improve stage Ⅱa rheumatoid arthritis. Besides AZD9056, other P2X7 inhibitors, such as CE-224535, have entered the clinical trial stage [120], but there is no research on their application in the treatment of kidney diseases.Brilliant Blue G (BBG) is a selective P2X7 receptor antagonist, which can reduce inflammation and fibrosis [121]. It was found that BBG could alleviate the kidney injury in Dahl salt-sensitive rats and LN mice [122].In addition, BBG can inhibit the infiltration of macrophages and fibroblasts, reduce the expression of inflammatory factors and collagen, inhibit cell apoptosis and promote the regeneration of renal tubular cells. However, the safety and clinical efficacy of BBG in renal diseases need to be studied.
5.4. Drugs targeting K+ channels
A study [123] found that glibenclamide combined with ATP-sensitive K+ channel could inhibit NLRP3. As an NLRP3 inflammasome inhibitor, glibenclamide can inhibit CKD and renal fibrosis induced by adenine in rats [124]. However, as a hypoglycemic agent, hypoglycemia and glucose metabolism disorder caused by glibenclamide limit its application in other diseases [125].
6. Conclusion
With the increasing incidence of metabolic diseases such as hypertension and diabetes and the aging of the social population, the incidence of chronic kidney disease (CKD) is increasing year by year, which seriously threatens people's lives and health. Therefore, how to prevent and treat CKD scientifically and effectively has become an urgent problem for clinicians. Drugs are the main means to delay the progress of CKD. Although with the development of modern medicine, the treatment of CKD has made great progress, the types of drugs that can be selected clinically to protect kidney and delay the progress of CKD are limited, and most of them are expensive, so it is urgent to find more cheap and efficient CKD drugs.
A large amount of evidence shows that there is a correlation between kidney diseases and inflammatory bodies, especially those containing NLRP3 in nucleotide-binding oligomeric domain-like receptor family. As a research hotspot, the NLRP3 inflammasome plays a significant role in inducing kidney inflammation and fibrosis.It is one of the most studied inflammasomes whose downstream pro-inflammatory molecules, IL-18 and IL-1β, play a crucial role in the pathogenesis of CKD. Although considerable advances have been made in understanding the NLRP3 inflammasome, its mechanisms are not entirely defined. The specific role of calcium signaling, mitochondrial dysfunction, and lysosome disruption in the NLRP3 activation is still controversial, requiring further verification [126,127].
Hence, the NLRP3 inflammasome-mediated inflammation participates in the development of CKD. During CKD, the NLRP3 inflammasome activation is the central mechanism in renal pathology, having an important role in injury and tissue remodeling. Thus, regulating autophagy, reducing ROS production, and inhibiting the NLRP3 inflammasome activation is a tool to alleviate renal inflammation.We could end the conclusion by emphasizing that because NLRP3 has pleiotropic effects on inflammation and yet unknown inflammation-independent roles, identifying its specific functions is paramount for establishing selective therapeutic strategies using this protein.Taken together, the NLRP3 inflammasome has great clinical significance as a promising target for the prevention and treatment of kidney diseases.
NLRP3 inflammasome is involved in the occurrence and development of kidney diseases, but its further mechanism and clinical application still need to be studied, and the research on the effect of NLRP3 inflammasome is still in its infancy. Understanding the related signal transduction pathway, regulatory mechanism and pathological significance is helpful to propose new strategies for prevention and treatment of kidney diseases. Compared with the currently used biological agents with larger molecules, the small molecule inhibitors that directly target NLRP3 inflammasome has the advantages of strong pertinence and low cost, and their toxicity is low because of their low drug concentration, so they show a good application prospect. Some drugs targeting NLRP3 inflammatory corpuscles and its downstream effector have made gratifying achievements in the treatment of other diseases, but there is still little research in kidney. These drugs may have potential value in treating CKD. However, it still takes a long time to transform the experimental data into clinical application, and the effectiveness and safety of these drugs in the treatment of kidney diseases need to be clarified.
Authors contributions
Gengzhen Huang and Yuerong Ma conceived the idea. Yingying Zhang analyzed data and literature. Gengzhen Huang wrote the manuscript. Zhang Yaodan edited the manuscript. Yuerong Ma validated the manuscript.
Availability of data and materials
Not applicable.
Ethics and dissemination
This study will not involve the individual patient and any ethical problems since its outcomes are based on published data.
Consent for publication
Authors are responsible for correctness of the statements provided in the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.81973732) and was funded by the Science and Technology Strategic Cooperation Project of Nanchong City (No.19SXHZ0181).
Declaration of competing interest
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to appreciate all anonymous reviewers for their insightful comments and constructive suggestions.We thank Bullet Edits Limited for linguistic editing and proofreading of the manuscript.
Contributor Information
Gengzhen Huang, Email: 53914005@qq.com.
Yaodan Zhang, Email: 363488093@qq.com.
Yingying Zhang, Email: yingyingzhang1117@163.com.
Yuerong Ma, Email: mayr666@163.com.
Abbreviations
- CKD
Chronic kidney disease
- ESRD
End-stage renal disease
- NLRP3
NLR family pyrin domain containing 3
- PAMPs
Pathogen-associated molecular patterns
- DAMPs
damage-associated molecular patterns
- ASC
apoptosis-associated speck-like protein
- ROS
reactive oxygen species
- LRR
leucine-rich repeat
- TXNIP
thioredoxin-interacting protein
- AngⅡ
Angiotensin Ⅱ
- HK-2
renal tubular epithelial cells
- NF-kB
nuclear factor kappa-B
- NEK7
NIMA-related kinase 7
- Interleukin-1β
IL-1β
- Interleukin-18
IL-18
- GFR
Glomerular filtration rate
References
- 1.Amadi C.E., Mbakwem A.C., Kushimo O.A., et al. Prevalence of positive chronic kidney Disease screening in professional male long haul drivers at risk of cardiovascular Disease in Lagos, Nigeria: a cross-section study. BMC Publ. Health. 2019;19:1032. doi: 10.1186/s12889-019-7328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al R.F., Stewart D., Fernandez-Llimos F., et al. Clinical pharmacy practice in the care of Chronic Kidney Disease patients: a systematic review. Int. J. Clin. Pharm. 2019;41:630–666. doi: 10.1007/s11096-019-00816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L., Wang F., Wang L., et al. Prevalence of chronic kidney disease in China:a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 4.Lin C.C., Hwang S.J. Patient-centered self-management in patients with chronic kidney disease: challenges and implications. Int. J. Environ. Res. Publ. Health. 2020;17(24):9443. doi: 10.3390/ijerph17249443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey A.S., Eckardt K.-U., Dorman N.M., et al. Nomenclature for kidney function and disease: executive summary and glossary from a kidney disease: improving global outcomes (KDIGO) consensus conference. J. Ren. Care. 2020;46:136. doi: 10.1053/j.jrn.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Wu X. Fujian Medical University; 2021. Study on Exercise Behavior and Influencing Factors of Patients with Chronic Kidney Disease. [DOI] [Google Scholar]
- 7.Chu C.D., Powe N.R., McCulloch C.E., et al. Trends in chronic kidney disease care in the US by race and ethnicity, 2012-2019. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo C.C., Chang C.M., Liu K.T., et al. Automation of the kidney function prediction and classification through ultrasound-based kidney imaging using deep learning. NPJ Digit Med. 2019;2:29. doi: 10.1038/s41746-019-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liyanage T., Ninomiya Toshiharu, Jha Vivekanand, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 10.Jha Vivekanand, Garcia-Garcia Guillermo, Kunitoshi Iseki, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 11.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to thera- peutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black L.M., Lever J.M., Agarwal A. Renal inflammation and fibrosis: a double-edged sword. J. Histochem. Cytochem. 2019;67:663–681. doi: 10.1369/0022155419852932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphreys B.D. Mechanisms of renal fibrosis. Annu. Rev. Physiol. 2018;80:309–326. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 14.Zhou T., Luo M., Cai W., et al. Runt-related transcription factor 1 (RUNX1) promotes TGF-β-induced renal tubular epithelial-to-mesenchymal transition (EMT) and renal fibrosis through the PI3K subunit p110δ. EBioMedicine. 2018;31:217–225. doi: 10.1016/j.ebiom.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin A., Tonelli M., Bonventre J., et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–1917. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 16.Nagashima R., Iyoda M. The roles of kidney-resident ILC2 in renal inflammation and fibrosis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.688647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullard A. NLRP3 inhibitors stoke anti-inflammatory ambitions. Nat. Rev. Drug Discov. 2019;18(6):405–407. doi: 10.1038/d41573-019-00086-9. [DOI] [PubMed] [Google Scholar]
- 18.Mulay S.R. Multifactorial functions of the inflammasome component NLRP3 in pathogenesis of chronic kidney diseases. Kidney Int. 2019;96(1):58–66. doi: 10.1016/j.kint.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Platnich J.M., Muruve D.A. NOD-like receptors and inflammasomes: a review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019;670:4–14. doi: 10.1016/j.abb.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Bai H., Yang B., Yu W., et al. Cathepsin B links oxidative stress to the activation of NLRP3 inflammasome. Exp. Cell Res. 2018;362(1):180–187. doi: 10.1016/j.yexcr.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Lee E.H., Shin J.H., Kim S.S., et al. Sinapic acid controls inflammation by suppressing NLRP3 inflammasome activation. Cells. 2021;10 doi: 10.3390/cells10092327. undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.M., Kim Y.G., Kim D.J., et al. Inflammasome-independent role of NLRP3 mediates mitochondrial regulation in renal injury. Front. Immunol. 2018;9:2563. doi: 10.3389/fimmu.2018.02563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Futosi K., Fodor S., Mócsai A. Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharm. 2013;17(4):1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 24.He W.T., Wan H., Hu L., et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016;16(7):407–420. doi: 10.1038/nri.2016.5. [DOI] [PubMed] [Google Scholar]
- 26.Feng H., Gu J., Gou F., et al. High glucose and lipopolysaccharide prime NLRP3 inflammasome via ROS/TXNIP pathway in mesangial cells. J. Diabetes Res. 2016;2016 doi: 10.1155/2016/6973175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H., Zhao L., Yue L., et al. Pterostilbene attenuates early brain injury following subarachnoid hemorrhage via inhibition of the NLRP3 inflammasome and Nox2-related oxidative stress. Mol. Neurobiol. 2017;54(8):5928–5940. doi: 10.1007/s12035-016-0108-8. [DOI] [PubMed] [Google Scholar]
- 28.Katsnelson M.A., Rucker L.G., Russo H.M., et al. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J. Immunol. 2015;194:3937–3952. doi: 10.4049/jimmunol.1402658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loock J., Lamprecht P., Timmann C., et al. Genetic predisposition (NLRP3 V198M mutation) for IL-1-mediated inflammation in a patient with Schnitzler syndrome. J. Allergy Clin. Immunol. 2010;125:500–502. doi: 10.1016/j.jaci.2009.10.066. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y.-K., Koppula S., Shim D.-W., et al. Arctium lappaInhibitory effect and mechanism of extract inflammasome activation. Evid. Based Complement Alternat. Med. 2018 doi: 10.1155/2018/6346734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossol M., Pierer M., Raulien N., et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang W., Bi J., Xu Q. Expression of NLRP3 inflammasome in chronic kidney disease and intervention mechanism of traditional Chinese medicine. Pharmacol. Clin. Tradit. Chin. Med. 2016;32(3):208–211. doi: 10.13412/j.cnki.zyyl.2016.03.062. [DOI] [Google Scholar]
- 33.Watanabe S., Usui-Kawanishi F., Karasawa T., et al. Glucose regulates hypoxia-induced NLRP3 inflammasome activation in macrophages. J. Cell. Physiol. 2020;235:7554–7566. doi: 10.1002/jcp.29659. [DOI] [PubMed] [Google Scholar]
- 34.Xiao Y., Huang Y., Wang H., et al. Thioredoxin-interacting protein mediates NLRP3 inflammasome activation involved in the susceptibility to ischemic acute kidney injury in diabetes. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/2386068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen Y., Liu Y., Tang T., et al. mrOS-TXNIP axis activates NLRP3 inflammasome to mediate renal injury during ischemic AKI. Int. J. Biochem. Cell Biol. 2018;98(3):43–53. doi: 10.1016/j.biocel.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Magupalli V.G., Negro R., Tian Y., et al. HDAC6 mediates an aggresome-like mechanism for NLRP3 and pyrin inflammasome activation. Science. 2020;369(6510) doi: 10.1126/science.aas8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra S.R., Mahapatra K.K., Behera B.P., et al. Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics. Int. J. Biochem. Cell Biol. 2021;136 doi: 10.1016/j.biocel.2021.106013. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang Y., Yasinta M., Hu C., et al. Mitochondrial dysfunction confers albumin-induced NLRP3 inflammasome activation and renal tubular injury. Am. J. Physiol. Ren. Physiol. 2015;308(8):F857–F866. doi: 10.1152/ajprenal.00203.2014. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y., He F., Li Y., et al. Effects of shizhifang on NLRP3 inflammasome activation and renal tubular injury in hyperuricemic rats. Evid. Based Complement Alternat. Med. 2017;2017 doi: 10.1155/2017/7674240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korolchuk V.I., Sarkar S., Fanto M. Autophagy in neurodegenerative diseases. J. Mol. Biol. 2020;432:2445–2448. doi: 10.3390/ijms21093369. [DOI] [PubMed] [Google Scholar]
- 41.Akther M., Haque M.E., Park J., et al. NLRP3 ubiquitination-A new approach to target NLRP3 inflammasome activation. Int. J. Mol. Sci. 2021;22(16):8780. doi: 10.3390/ijms22168780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia L., Xu Z., Zhou X., et al. Impaired autophagy increases susceptibility to endotoxin-induced chronic pancreatitis. Cell Death Dis. 2020;11:889. doi: 10.1038/s41419-020-03050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenoir O., Jasiek M., Hénique C., et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy. 2015;11(7):1130–1145. doi: 10.1080/15548627.2015.1049799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie X., Zhang Y., Wang Z., et al. ATM at the crossroads of reactive oxygen species and autophagy. Int. J. Biol. Sci. 2021;17:3080–3090. doi: 10.7150/ijbs.63963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amaral E.P., Riteau N., Moayeri M., et al. Mycobacterium tuberculosisLysosomal cathepsin release is required for NLRP3-inflammasome activation by in infected macrophages. Front. Immunol. 2018;9:1427. doi: 10.3389/fimmu.2018.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leu W.-J., Chu J.-C., Hsu J.-L., et al. Chalcones display anti-NLRP3 inflammasome activity in macrophages through inhibition of both priming and activation steps-structure-activity-relationship and mechanism studies. Molecules. 2020;25(24):5960. doi: 10.3390/molecules25245960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y., Gao C., Vong C., et al. Rhein regulates redox-mediated activation of NLRP3 inflammasomes in intestinal inflammation through macrophage-activated crosstalk. Br. J. Pharmacol. 2022;179(9):1978–1997. doi: 10.1111/bph.15773. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y., Li X., Boini K.M., et al. Endothelial Nlrp3 inflammasome activation associated with lysosomal destabilization during coronary arteritis. Biochim. Biophys. Acta. 2015;1853:396–408. doi: 10.1016/j.bbamcr.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu J., Lu L., Li L. NEK7: a novel promising therapy target for NLRP3-related inflammatory diseases. Acta Biochim. Biophys. Sin. 2016;48:966–968. doi: 10.1093/abbs/gmw080. [DOI] [PubMed] [Google Scholar]
- 50.He Y., Zeng M.Y., Yang D., et al. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang T., Li P., Zhou X., et al. The E3 ubiquitin ligase TRIM65 negatively regulates inflammasome activation through promoting ubiquitination of NLRP3. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.741839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi H., Wang Y., Li X., et al. NLRP3 activation and mitosis are mutually exclusiveevents coordinated by Nek7, a new inflammasome component. Nat. Immunol. 2016;17(3):250–258. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.An X., Zhang Y., Cao Y., et al. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 2020;12(5):1516. doi: 10.3390/nu12051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao B., Wang Y., Xiao Y., et al. Effect of TXNIP on hyperglycemic hypoxia and reoxygenation injury of human renal tubular epithelial cells. J. Trop. Med. 2018;18(4):473–475. doi: 10.3969/j.issn.1672-3619.2018.04.013. [DOI] [Google Scholar]
- 55.Mo Y., Yang Y., Cui L. Txnip-mediated oxidative stress and its mechanism in disease. Chin. Pharmacol. Bull. 2018;34(1):16–19. doi: 10.3969/j.issn.1001-1978.2018.01.005. [DOI] [Google Scholar]
- 56.Li G. Hebei Medical University; 2019. The Role of TXNIP in Renal Ischemia-Reperfusion Injury in Mice. [DOI] [Google Scholar]
- 57.Ying C., Zhou Z., Dai J., et al. Activation of the NLRP3 inflammasome by RAC1 mediates a new mechanism in diabetic nephropathy. Inflamm. Res. 2022;71:191–204. doi: 10.1007/s00011-021-01532-4. [DOI] [PubMed] [Google Scholar]
- 58.El-Deeb O.S., Atef M.M., Hafez Y.M. The interplay between microbiota-dependent metabolite trimethylamine N-oxide, Transforming growth factor β/SMAD signaling and inflammasome activation in chronic kidney disease patients: a new mechanistic perspective. J. Cell. Biochem. 2019;120(9):14476–14485. doi: 10.1002/jcb.28707. [DOI] [PubMed] [Google Scholar]
- 59.Guo H., Callaway J.B., Ting J.P.-Y. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krishnan S.M., Ling Y.H., Huuskes B.M., et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019;115(4):776–787. doi: 10.1093/cvr/cvy252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen Y., Pan M.M., Lv L.L., et al. Artemisinin attenuates tubulointerstitial inflammation and fibrosis via the NF-κB/NLRP3 pathway in rats with 5/6 subtotal nephrectomy. J. Cell. Biochem. 2019;120(3):4291–4300. doi: 10.1002/jcb.27714. [DOI] [PubMed] [Google Scholar]
- 62.Zhao M., Bai M., Ding G., et al. Angiotensin II stimulates the NLRP3 inflammasome to induce podocyte injury and mitochondrial dysfunction. Kidney Dis. 2018;4(2):83–94. doi: 10.1159/000488242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lian D., Lai J., Wu Y., et al. Cathepsin B-mediated NLRP3 inflammasome formation and activation in angiotensin II -induced hypertensive mice: role of macrophage digestion dys function. Cell. Physiol. Biochem. 2018;50(4):1585–1600. doi: 10.1159/000494656. [DOI] [PubMed] [Google Scholar]
- 64.Kaneko Y., Sano M., Seno K., et al. Olive leaf extract (OleaVita) suppresses inflammatory cytokine production and NLRP3 inflammasomes in human placenta. Nutrients. 2019;11(5):970. doi: 10.3390/nu11050970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun X. Anhui Medical University; 2020. To Explore the Role of Bruton Tyrosine Kinase Regulating the Activation of NLRP3 Inflammatory Bodies in the Inflammatory Mechanism of Diabetic Nephropathy Based on Macrophages. [DOI] [Google Scholar]
- 66.Tian Y. Hebei Medical University; 2020. Study on the Protective Effect and Mechanism of Intermediate Filament Protein Nestin in Podocyte Injury of Lupus Nephritis. [DOI] [Google Scholar]
- 67.Zhang C. China Medical University; 2019. MCC950 and Fermented Cordyceps Powder Down-Regulate NLRP3/Caspase-1/IL-1β Pathway to Improve Diabetic Kidney Injury. [DOI] [Google Scholar]
- 68.Zhang K., Fan C., Cai D., et al. Contribution of TGF-beta-mediated NLRP3-HMGB1 activation to tubul- ointerstitial fibrosis in rat with angiotensin II-induced chronic kidney disease. Front. Cell Dev. Biol. 2020;8:1. doi: 10.3389/fcell.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ling L., Yang M., Ding W., Gu Y. Ghrelin attenuates UUO-induced renal fibrosis via attenuation of Nlrp3 inflammasome and endoplasmic reticulum stress. Am. J. Transl. Res. 2019;11(1):131–141. [PMC free article] [PubMed] [Google Scholar]
- 70.Guo H., Bi X., Zhou P., Zhu S., Ding W. NLRP3 deficiency attenuates renal fibrosis and ameliorates mitochondrial dysfunction in a mouse unilateral ureteral obstruction model of chronic kidney disease. Mediat. Inflamm. 2017;2017 doi: 10.1155/2017/8316560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choucry M.A., Khalil M.N.A., El Awdan S.A. Protective action of Crateva nurvala Buch. Ham extracts against renal ischaemia reperfusion injury in rats via antioxidant and anti-inflammatory activities. J. Ethnopharmacol. 2018;214:47–57. doi: 10.1016/j.jep.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 72.Fan J., Chen Y., Yang D., et al. Multi-walled carbon nanotubes induce IL-1β secretion by activating hemich- annels-mediated ATP release in THP-1 macrophages. Nanotoxicology. 2020;14:929–946. doi: 10.1080/17435390.2020.1777476. [DOI] [PubMed] [Google Scholar]
- 73.Zhu Y., Liang Z., Ren Z., et al. NLRP3 inflammasome-mediated inflammatory reaction is involved in kidney injury and abnormal lipid metabolism caused by diabetes. J. Chin. Pathophysiol. 2020;36(1):53–58. doi: 10.3969/j.issn.1000-4718.2020.01.008. [DOI] [Google Scholar]
- 74.Feng H., Gu J., Gou F., et al. High glucose and lipopolysaccharide prime NLRP3 inflammasome via ROS/TXNIP pathway in mesangial cells. J. Diabetes Res. 2016;2016 doi: 10.1155/2016/6973175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Samra Y.A., Said H.S., Elsherbiny N.M., et al. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: role of NF-κB and NLRP3 inflammasome. Life Sci. 2016;157:187–199. doi: 10.1016/j.lfs.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 76.Wang S., Zhao X., Yang S., et al. Salidroside alleviates high glucose-induced oxidative stress and extracellular matrix accumulation in rat glomerular mesangial cells by the TXNIP-NLRP3 inflammasome pathway. Chem. Biol. Interact. 2017;278:48–53. doi: 10.1016/j.cbi.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 77.Hsu Y.H., Zheng C.M., Chou C.L., et al. Therapeutic effect of endothelin-converting enzyme inhibitor on chronic kidney disease through the inhibition of endoplasmic reticulum stress a nd the NLRP3 inflammasome. Biomedicines. 2021;9(4):398. doi: 10.3390/biomedicines9040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chu J.-Q., Gao F.F., Wu W., et al. Expression profiles of NOD-like receptors and regulation of NLRP3 inflammasome activation in Toxoplasma gondii-infected human small intestinal epithelial cells. Parasites Vectors. 2021;14:153. doi: 10.1186/s13071-021-04666-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim S.-K., Park K.-Y., Choe J.-Y. Toll-like receptor 9 is involved in NLRP3 inflammasome activation and IL-1β production through monosodium urate-induced mitochondrial DNA. Inflammation. 2020;43:2301–2311. doi: 10.1007/s10753-020-01299-6. [DOI] [PubMed] [Google Scholar]
- 80.Ruan Y., Qiu X., Lv Y.-D., et al. Kainic acid Induces production and aggregation of amyloid β-protein and memory deficits by activating inflammasomes in NLRP3- and NF-κB-stimulat ed pathways. Aging (Albany NY) 2019;11:3795–3810. doi: 10.18632/aging.102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ismael S., Nasoohi S., Yoo A., et al. Tissue plasminogen activator promotes TXNIP-NLRP3 inflammasome activation after hyperglycemic stroke in mice. Mol. Neurobiol. 2020;57:2495–2508. doi: 10.1007/s12035-020-01893-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang L., Gong X., Liu H. The role of NLRP3 inflammasomes in EMT of mouse renal tubular epithelial cells induced by high glucose. J. Changzhi Med. Coll. 2017;31(1):16–19. doi: 10.3969/j.issn.1006-0588.2017.01.005. [DOI] [Google Scholar]
- 83.Jung G.S., Hwang Y.J., Choi J.H., Lee K.M. Lin28a attenuates TGF-β-induced renal fibrosis. BMB Rep. 2020;53(11):594–599. doi: 10.5483/BMBRep.2020.53.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakai Wada T. Revisiting inflammation in diabetic nephropathy: the role of the Nlrp3 inflammasome in glomerular resident cells. Kidney Int. 2015;87:12–14. doi: 10.1038/ki.2014.322. [DOI] [PubMed] [Google Scholar]
- 85.Shahzad K., Bock F., Dong W., et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015;87(1):74–84. doi: 10.1038/ki.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du L., Wang J., Chen Y., et al. Novel biphenyl diester derivative AB-38b inhibits NLRP3 inflammasome through Nrf2 activation in diabetic nephropathy. Cell Biol. Toxicol. 2020;36(3):243–260. doi: 10.1007/s10565-019-09501-8. [DOI] [PubMed] [Google Scholar]
- 87.An X., Zhang Y., Cao Y., Chen J., Qin H., Yang L. Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 2020;12(5):1516. doi: 10.3390/nu12051516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang C., Hou X.X., Rui H.L., et al. Artificially cultivated ophiocordyceps sinensis alleviates diabetic nephropathy and its podocyte injury via inhibiting P2X7R expression and NLRP3 inflammasome activation. J. Diabetes Res. 2018;2018 doi: 10.1155/2018/1390418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao Y., Guo Y., Jiang Y., Zhu X., Liu Y., Zhang X. Mitophagy regulates macrophage phenotype in diabetic nephropathy rats. Biochem. Biophys. Res. Commun. 2017;494(1–2):42–50. doi: 10.1016/j.bbrc.2017.10.088. [DOI] [PubMed] [Google Scholar]
- 90.Hou Y., Lin S., Qiu J., et al. NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem. Biophys. Res. Commun. 2020;521(3):791–798. doi: 10.1016/j.bbrc.2019.10.194. [DOI] [PubMed] [Google Scholar]
- 91.Tsai Y.L., Hua K.F., Chen A., et al. NLRP3 inflammasome: pathogenic role and potential therapeutic target for IgA nephropathy. Sci. Rep. 2017;7 doi: 10.1038/srep41123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng W., Pei G.Q., Tang Y., Tan L., Qin W. IgA1 deposition may induce NLRP3 expression and macrophage transdifferentiation of podocyte in IgA nephropathy. J. Transl. Med. 2019;17(1):406. doi: 10.1186/s12967-019-02157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng W., Pei G.-Q., Tang Y., et al. IgA1 deposition may induce NLRP3 expression and macrophage transdifferentiation of podocyte in IgA nephropathy. J. Transl. Med. 2019;17:406. doi: 10.1186/s12967-019-02157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zahran R., Ghozy A., Elkholy S.S., et al. Combination therapy with melatonin, stem cells and extracellular vesicles is effective in limiting renal ischemia-reperfusion injury in a rat model. Int. J. Urol. 2020;27(11):1039–1049. doi: 10.1111/iju.14345. [DOI] [PubMed] [Google Scholar]
- 95.Zheng L., Tang X., Lu M., et al. microRNA-421-3p prevents inflammatory response in cerebral ischemia/reperfusion injury through targeting m6A Reader YTHDF1 to inhibit p65 mRNA translation. Int. Immunopharm. 2020;88 doi: 10.1016/j.intimp.2020.106937. [DOI] [PubMed] [Google Scholar]
- 96.Artiles M.A., Burgos R.F.J., Álvarez N.M., et al. In situComparison of preservation techniques for kidneys from donors after circulatory death: a systematic review and meta-analysis. Transl. Androl. Urol. 2021;10:3286–3299. doi: 10.21037/tau-21-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karaba A.H., Figueroa A., Massaccesi G., et al. Herpes simplex virus type 1 inflammasome activation in proin- flammatory human macrophages is dependent on NLRP3, ASC, and caspase-1. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee K., Gusella G.L., He J.C. Epithelial proliferation and cell cycle dysregulation in kidney injury and disease. Kidney Int. 2021;100:67–78. doi: 10.1016/j.kint.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu M., Han W., Song S., et al. NLRP3 deficiency ameliorates renal inflammation and fibrosis in diabetic mice. Mol. Cell. Endocrinol. 2018;478:115–125. doi: 10.1016/j.mce.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 100.Kim Y.G., Kim S.-M., Kim K.-P., et al. The role of inflammasome-dependent and inflammasome-independent NLRP3 in the kidney. Cells. 2019;8(11):1389. doi: 10.3390/cells8111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Foresto-Neto O., Ávila V.F., Arias S.C.A., et al. NLRP3 inflammasome inhibition ameliorates tubulointerstitial injury in the remnant kidney model. Lab. Invest. 2018;98(6):773–782. doi: 10.1038/s41374-018-0029-4. [DOI] [PubMed] [Google Scholar]
- 102.Faustino V.D., Arias S.C.A., Ferreira Ávila V., et al. Simultaneous activation of innate and adaptive immunity participates in the development of renal injury in a model of heavy proteinuria. Biosci. Rep. 2018;38(4) doi: 10.1042/BSR20180762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sharma M., Naura A.S., Singla S.K. Modulatory effect of 4-phenyl butyric acid on hyperoxaluria-induced renal injury and inflammation. Mol. Cell. Biochem. 2019;451:185–196. doi: 10.1007/s11010-018-3405-x. [DOI] [PubMed] [Google Scholar]
- 104.Lan Z., Chen L., Feng J., et al. Mechanosensitive TRPV4 is required for crystal-induced inflammation. Ann. Rheum. Dis. 2021;80:1604–1614. doi: 10.1136/annrheumdis-2021-220295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mulay S.R., Kumar S.V., Lech M., et al. How kidney cell death induces renal Necroinflammation. Semin. Nephrol. 2016;36:162–173. doi: 10.1016/j.semnephrol.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 106.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang C., Zhu X., Li L., et al. A small molecule inhibitor MCC950 ameliorates kidney injury in diabetic nephropathy by inhibiting NLRP3 inflammasome activation. Diabetes Metab. Syndr. Obes. 2019;12:1297–1309. doi: 10.2147/DMSO.S199802. Published 2019 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krishnan S.M., Ling Y.H., Huuskes B.M., et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019;115(4):776–787. doi: 10.1093/cvr/cvy252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ludwig-Portugall I., Bartok E., Dhana E., et al. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 2016;90(3):525–539. doi: 10.1016/j.kint.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 110.Cornelius D.C., Travis O.K., Tramel R.W., et al. NLRP3 inflammasome inhibition attenuates sepsis-induced platelet activation and prevents multi-organ injury in cecal-ligation puncture. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234039. Published 2020 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li S., Lin Q., Shao X., et al. NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp. Cell Res. 2019;383(1) doi: 10.1016/j.yexcr.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 112.Huang Y., Jiang H., Chen Y., et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018;10(4) doi: 10.15252/emmm.201708689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen S., Wang Y., Pan Y., et al. Novel role for tranilast in regulating NLRP3 ubiquitination, vascular inflamma- tion, and atherosclerosis. J. Am. Heart Assoc. 2020;9(12) doi: 10.1161/JAHA.119.015513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Said E., Elkashef W.F., Abdelaziz R.R. Tranilast ameliorates cyclophosphamide-induced lung injury and nephrotoxi- city. Can. J. Physiol. Pharmacol. 2016;94(4):347–358. doi: 10.1139/cjpp-2015-0070. [DOI] [PubMed] [Google Scholar]
- 115.Youm Y.H., Nguyen K.Y., Grant R.W., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Anders H.J., Suarez-Alvarez B., Grigorescu M., et al. The macrophage phenotype and inflammasome component NLRP3 contributes to nephrocalcinosis-related chronic kidney disease independent from IL-1-mediated tissue injury. Kidney Int. 2018;93(3):656–669. doi: 10.1016/j.kint.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 117.Jiang H., He H., Chen Y., et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disor- ders. J. Exp. Med. 2017;214(11):3219–3238. doi: 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pan L.L., Liang W., Ren Z., et al. Cathelicidin-related antimicrobial peptide protects against ischaemia reperfusion-induced acute kidney injury in mice. Br. J. Pharmacol. 2020;177(12):2726–2742. doi: 10.1111/bph.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wannamaker W., Davies R., Namchuk M., et al. (S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18. J. Pharmacol. Exp. Therapeut. 2007;321(2):509–516. doi: 10.1124/jpet.106.111344. [DOI] [PubMed] [Google Scholar]
- 120.Marques C.C., Castelo-Branco M.T., Pacheco R.G., et al. Prophylactic systemic P2X7 receptor blockade prevents experimental colitis. Biochim. Biophys. Acta. 2014;1842(1):65–78. doi: 10.1016/j.bbadis.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 121.Ji X., Naito Y., Hirokawa G., et al. P2X(7) receptor antagonism attenuates the hypertension and renal injury in Dahl salt-sensitive rats. Hypertens. Res. 2012;35(2):173–179. doi: 10.1038/hr.2011.153. [DOI] [PubMed] [Google Scholar]
- 122.Pereira J.M.S., Barreira A.L., Gomes C.R., et al. Brilliant blue G, a P2X7 receptor antagonist, attenuates early phase of renal inflammation, interstitial fibrosis and is associated with renal cell proliferation in ureteral obstruction in rats. BMC Nephrol. 2020;21(1):206. doi: 10.1186/s12882-020-01861-2. Published 2020 May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamkanfi M., Mueller J.L., Vitari A.C., et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009;187(1):61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Diwan V., Gobe G., Brown L. Glibenclamide improves kidney and heart structure and function in the adenine-diet model of chronic kidney disease. Pharmacol. Res. 2014;79:104–110. doi: 10.1016/j.phrs.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 125.Ozaki E., Campbell M., Doyle S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J. Inflamm. Res. 2015;8:15–27. doi: 10.2147/JIR.S51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ding Y., Hu R. Research progress of activation and regulation mechanism of NLRP3 inflammatory body. Pharmaceut. Prog. 2018;42(4):294–302. doi: 10.27204/d.cnki.glzhu.2017.000067. [DOI] [Google Scholar]
- 127.Yuan Z., Li J. Research progress of NLRP3 inflammatory corpuscles in renal fibrosis. Guangxi Med. Sci. 2020;42(3):338–341. doi: 10.11675/j.issn.0253-4304.2020.03.23. [DOI] [Google Scholar]
- 128.Zhao N., Li C.C., Di B., Xu L.L. Recent advances in the NEK7-licensed NLRP3 inflammasome activation: mechani- sms, role in diseases and related inhibitors. J. Autoimmun. 2020;113 doi: 10.1016/j.jaut.2020.102515. [DOI] [PubMed] [Google Scholar]
- 129.Sharif H., Wang L., Wang W.L., et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570(7761):338–343. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han Y., Xu X., Tang C., et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018;16:32–46. doi: 10.1016/j.redox.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dai X., Liao R., Liu C., et al. Epigenetic regulation of TXNIP-mediated oxidative stress and NLRP3 inflammasome activation contributes to SAHH inhibition-aggravated diabetic nephropathy. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang A., Xing J., Xia T., et al. EphA2 phosphorylates NLRP3 and inhibits inflammasomes in airway epithelial cells. EMBO Rep. 2020;21(7) doi: 10.15252/embr.201949666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.