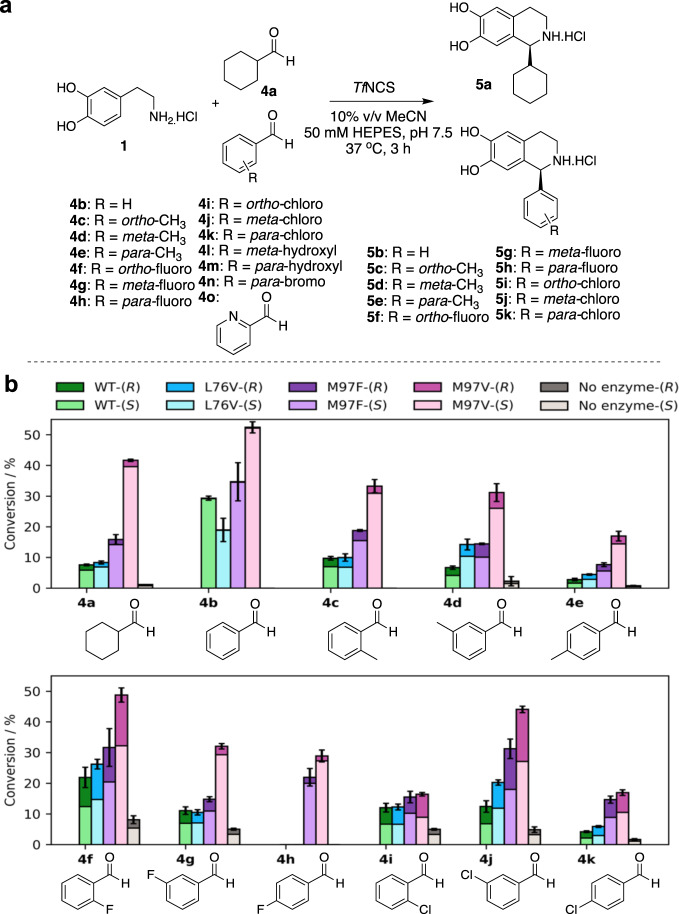
Fig. 3. Initial TfNCS-catalysed reactions between dopamine and aldehydes 4a–o.
a Reactions performed between dopamine (1) and aldehydes (4a–k). Reactions with aldehydes 4l–o were performed using 0.5 mg mL−1 final concentration of Δ29TfNCS-M97V. b Conversions of reactions between dopamine (1) and benzaldehyde derivatives (4a–k). WT-TfNCS or active site mutants of TfNCS were used as the reaction catalyst with 0.2 mg mL−1 enzyme. Samples were prepared by workup method 1, conversions were determined by monitoring product formation against standards (Supplementary Figs. 24–26 and Supplementary Methods) by analytical achiral HPLC (method 1). Product enantiomeric purities are given by indicating the amounts of R- and S-product generated and were determined by chiral HPLC analysis. Reactions were performed in triplicate and standard deviations reported.