Abstract
Background
Alzheimer’s disease (AD) is a common progressive neurodegenerative disease characterized by memory impairments, and there is no effective therapy. Neural stem/progenitor cell (NSPC) has emerged as potential novel therapy for AD, and we aim to explore whether neural stem/progenitor cell therapy was effective for rodent models of AD.
Methods
We searched PubMed, Embase, Cochrane Library and Web of Science up to December 6, 2022. The outcomes included cognitive function, pathological features and BDNF. The GetData Graph Digitizer software (version 2.26) was applied to extract numerical values, and RevMan 5.3 and Stata 16 were used to analyze data. The SYRCLE risk of bias tool was used to assess study quality.
Results
We evaluated 22 mice studies and 8 rat studies. Compared to control groups, cognitive function of NSPC groups of both mice studies (SMD = − 1.96, 95% CI − 2.47 to − 1.45, I2 = 75%, P < 0.00001) and rat studies (SMD = − 1.35, 95% CI − 2.11 to − 0.59, I2 = 77%, P = 0.0005) was apparently improved. In mice studies, NSPC group has lower Aβ deposition (SMD = − 0.96, 95% CI − 1.40 to − 0.52, P < 0.0001) and p-tau level (SMD = − 4.94, 95% CI − 7.29 to − 2.95, P < 0.0001), higher synaptic density (SMD = 2.02, 95% CI 0.50–3.55, P = 0.009) and BDNF (SMD = 1.69, 95% CI 0.61–2.77, P = 0.002). Combined with nanoformulation (SMD = − 1.29, 95% CI − 2.26 to − 0.32, I2 = 65%, P = 0.009) and genetically modified (SMD = − 1.29, 95% CI − 1.92 to − 0.66, I2 = 60%, P < 0.0001) could improve the effect of NSPC. In addition, both xenogeneic and allogeneic transplant of NSPC could reverse the cognitive impairment of AD animal models.
Conclusions
Our results suggested that NSPC therapy could improve the cognitive function and slow down the progression of AD. Due to the limitations of models, more animal trials and clinical trials are needed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-022-03231-1.
Keywords: Alzheimer disease, Neural stem/progenitor cell, Rodent models, Systematic review and meta-analysis
Introduction
Alzheimer disease (AD) is a common, progressive, and devastating neurodegenerative disease. The pathological features of the disease are the presence of extracellular amyloid-β (Aβ)-containing senile plaques and intracellular hyperphosphorylated tau-containing neurofibrillary tangles (NFT), neuroinflammation, synaptic loss and neuronal death, neocortical atrophy and the progressive deterioration of cognitive function [1, 2]. AD can be divided into familial Alzheimer disease (FAD) and sporadic AD (SAD) among the genetic factors. Most patients with Alzheimer’s disease (> 95%) have the sporadic form, which is characterized by a late onset (80–90 years of age) and is the consequence of the failure to clear the amyloid-β (Aβ) peptide from the interstices of the brain [3]. Familial Alzheimer’s disease (FAD) presents basic similarities to sporadic AD, but with important differences. Onset is in mid-life or earlier, and the genetics follows a dominant Mendelian pattern, with 100% penetrance in most pedigrees [4]. The pathogenesis of AD is complex, involving multiple molecular signaling pathways. Cholinergic deficiency, amyloid beta (Aβ) toxicity, tau protein hyperphosphorylation, synaptic dysfunction, oxidative stress, and neuroinflammation, were proposed to be responsible for AD development [5]. In 2018, Alzheimer’s Disease International estimated a dementia prevalence of about 50 million people worldwide, projected to triple in 2050, with two-thirds living in low-income and middle-income countries.
Today, only five drugs have been approved by the FDA for AD treatment: donepezil, rivastigmine, galantamine, tacrine and memantine. The first four drugs are acetylcholinesterase inhibitors (AChEIs), while the last one is an N-methyl-d-aspartate receptor (NMDAR) antagonist [6]. Clinical studies show some other approaches to AD, such as acupuncture, behavioral training and brain stimulation, including deep brain stimulation (DBS) [7], repetitive transcranial magnetic stimulation (rTMS) [8] and transcranial electrical stimulation (tDCS and tACS) [8, 9]. But current treatments are unable to achieve satisfactory therapeutic outcomes, new treatments are urgently needed.
In recent years, stem cell therapy has received growing attention as a potential regenerative therapy for neurodegenerative diseases including AD due to regeneration of neural tissue, stabilizing the neuronal networks, providing neurotrophic support and alleviating neurodegeneration at different neuronal circuitry levels [10]. In clinical trials, researchers are conducting the safety and efficacy of Mesenchymal Stem Cells and Autologous Adipose Tissue Derived Mesenchymal Stem Cells. Phase I clinical trials of human umbilical cord blood derived mesenchymal stem cells and Longeveron Mesenchymal Stem Cells preliminary prove that MSC therapy was feasible, relatively and sufficiently safe and well tolerated [11, 12]. As for other animal models, there are more types of stem cells—induced pluripotent stem cells (iPSCs), neural stem cells (NSCs), mesenchymal stem cells (MSCs) and embryonic stem cells (ESCs). Neural stem/progenitor cells (NSPCs) are the multipotent stem cells that are capable of proliferation, self-renewal and generation of new neurons, astrocytes and oligodendrocytes [13]. NSPCs were used for some animal models, which have evaluated the safety and effectiveness of NSPC therapy. But there is no meta-analysis to evaluate the efficacy and synthesize evidence of NSPC therapy in AD models. Therefore, the aim of this systematic review and meta-analysis is to assess the efficacy of NSPC therapy of experimental AD rodents, and our study will provide support for clinical treatment of NSPC for AD.
Methods
Data sources and search strategy
Four database (PubMed, Embase, Web of Science and Cochrane Library) were searched for experimentally controlled studies of the effect of NSPC therapies on AD models from their inception to December 6, 2022. The search strategy used a combination of terms from medical subject headings (MeSH) and free-text keywords. The subject headings were "Alzheimer Disease" AND "Neural Stem Cells" AND "Mice" OR "Rats." Combined with free words: (Alzheimer Dementia OR Dementia, Alzheimer OR Alzheimer’s Disease OR Alzheimer Syndrome) AND (Neural Stem Cell OR Neural Progenitor Cell OR neural stem/progenitor cell) AND (mouse OR rat). Manual search and other methods were used to identify other relevant articles. Information of detailed search strategy is shown in Additional file 1: Table S1.
Criteria for consideration and extraction
Inclusion criteria (1) AD mice/rats treated by NSPCs; (2) Studies provided data about MWM or Aβ level; (3) Studies were published in English.
Exclusion criteria (1) No in vivo texting; (2) Review or conference abstract; (3) No NSPC group or control group; (4) No outcome or incomplete data.
Study selection
The literature retrieved from each database was imported into the NoteExpress, and the duplicated papers were removed. Then, titles and abstracts were scrutinized to determine the eligible studies after excluding the irrelevant articles. Then, full-text papers were obtained reviewed for the final eligibility according to the inclusion and exclusion criteria stated above. Two researchers independently select the studies, and a third researcher was consulted to resolve any disagreements.
Data extraction and quality assessment
Two researchers independently evaluated article quality and extracted data, and disagreements were addressed by discussion with a third reviewer. We extracted the following data from each study: first author, year, location, sex, species, weight and year of animals, method of AD model induction, source of NSPC, dose of cells, way and location of administrated, groups of trials, assessment time, immunosuppression or not and outcome. If data were only shown by graphs, the GetData Graph Digitizer software (version 2.26) was applied to extract numerical values. When SD was not reported, it was calculated by √N × SE, and N means the sample size. If the required information was not obtained, the study was deleted. The SYRCLE risk of bias tool was used to evaluate the quality of animal studies [14].
Statistical analysis
Cochrane Collaboration Software RevMan 5.3 and Stata 16 were used to analyze data. The combined effect size was calculated as standardized mean difference (SMD) with 95% confidence interval (CI) between treatment group and control group. Heterogeneity was statistically evaluated by I2value, I2 ≤ 50% indicated homogeneity and fixed-effect models were employed, or random-effect models were used instead. Subgroup analyses were performed to indicate statistical significance. Publication bias was investigated by visual inspection of funnel plots. All tests were two-sided, and P < 0.05 was considered to indicate statistical significance.
Results
Search results
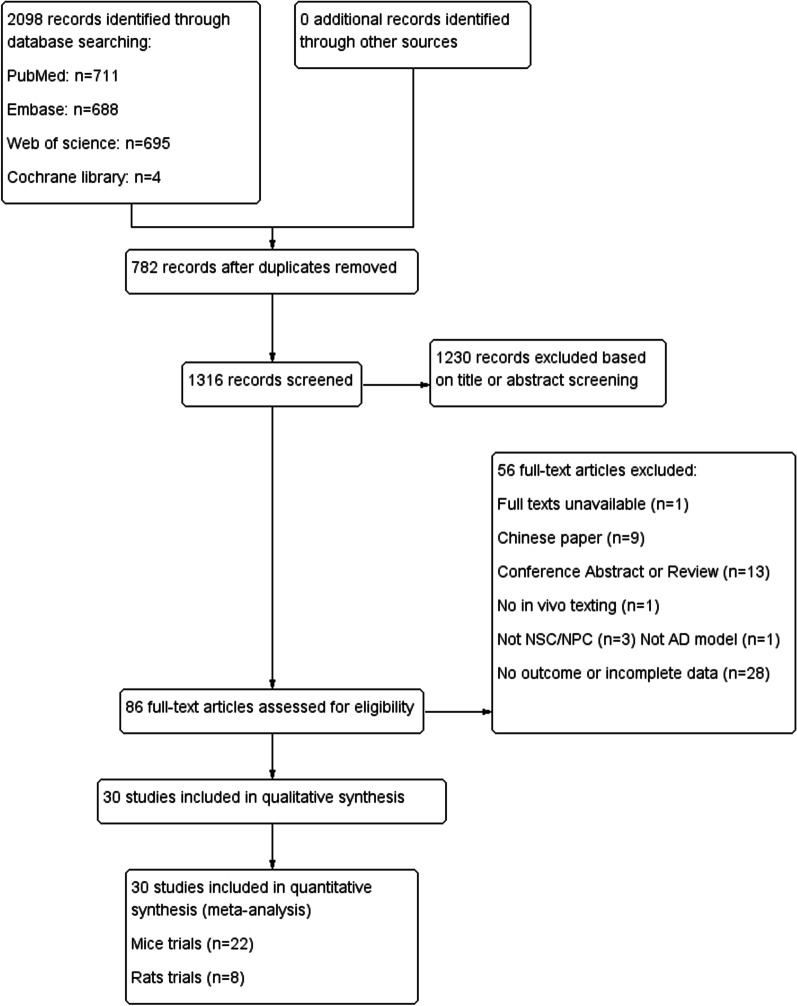
A total of 2098 articles were initially retrieved from 4 databases, and 1316 records were obtained after removing 782 duplicates. Then after screening titles and abstracts, 86 full-text articles were assessed for eligibility. Fifty-six of them were excluded because of full text unavailable, Chinese paper, conference abstract or review, no in vivo texting, no NSPC or AD model and no outcome or incomplete data. Finally, 22 mice trials [15–36] and 8 rat trials [37–44] were selected (Fig. 1). Funnel plots were used to evaluate publication bias (Additional file 2: Fig. S1).
Fig. 1.
PRISM flowchart of study selection process. A total of 2098 records were retrieved; after application of the inclusion criteria, 22 mice studies and 8 rat studies were included
Study characteristics and quality
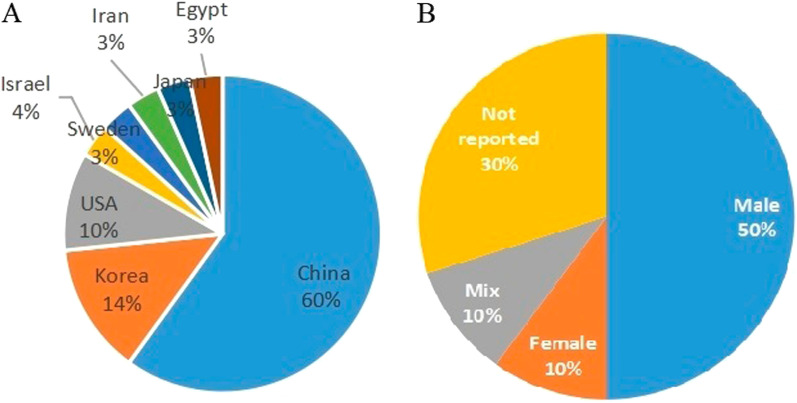
Of 30 studies, 22 were mice models and 8 were rat models. The location of studies included China, Korea, the USA, Israel, Sweden, Iran, Japan and Egypt (Fig. 2a). The gender of the experimental animal of all studies included only male, or only female, or the mixed, except for 9 studies with no statements (Fig. 2b). Of all mice models, APPswe/PS1dE9 mice were used in 11 studies, Tg2576 mice and APP/PS1/tau 3 × Tg AD mice were used in 3 studies, SAMP8 mice were used in 2 studies, and Tg-tau mice, NSE/APPsw transgenic mice and ICR mice infused with ibotenic acid were used in other studies. Sixteen studies used mice NSPC, 6 studies used human NSPC, and 3 studies used immunosuppression. As for rat model, 6 studies used SD rats and 2 used Wistar rats, while the method of AD is different, such as infusing AF64A solution, Aβ, okadaic acid (OA), IgG-saporin, ibotenic (IBO) acid and nucleus basalis of Meynert (nbM) lesioning. Four studies used rat NSPC, other 4 studies including 2 used human NSPC and 2 used mice NSPC, and 3 of them used immunosuppression. Of all studies, there were 14 studies combined with other treatment methods. In almost all studies, NSPCs were stereotactically transplanted, only 1 was intranasally transplanted and 3 studies were intra-cerebroventricular injection. Information on study characteristics, study quality and publication bias is shown in Tables 1, 2, Additional files 3: Table S2, and 2: Fig. S1.
Fig. 2.
Mapping of study characteristics included in the systematic review. A Location of studies. B Gender of animals
Table 1.
Characteristics of mice trials
| References | Location | Animal sex | Animal species | Animal year | Group | Type and source of NSPC | Way of administrated | Delivered location | Dose of NSPC | Immunosuppression | Assessment time | Parameter |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Huang [15] | China | Female | APPswe/PS1dE9 mice | 9 months |
AD + PBS AD + NSC AD + nanoformulation-NSC AD + NEP–NSC |
Mice NSC from hippocampus | Stereotactically transplanted | Hippocampus | 1 × 105 | N | 1 month 6 months | ①② |
| Wu [16] | China | NA | Tg2576 mice | 16 months |
Tg + Vehicle Tg + NSC Tg + BDNF-NSC |
Mice NSC from postnatal day 1 hippocampus | Stereotactically transplanted | Hippocampus | 1 × 105 | N | 8 weeks | ①②④⑥ |
| Lee [17] | Korea | male | ICR mice | NA |
IBO acid + NSC (n = 9) IBO acid + PBS (n = 7) IBO acid + NGF-NSC (n = 9) |
Human NSC from 14-week fetal brain | Stereotactically transplanted | Cortex | 2 × 105 | N | 4 weeks | ① |
| McGinley [18] | USA | Male | APPswe/PS1dE9 mice | 12 weeks |
AD + vehicle (n = 10) AD + NSC (n = 10) |
Human cortex-derived NSC | Stereotactically transplanted | Fimbria fornix | 1.8 × 105 | Y | 16 weeks | ①②④⑤ |
| Zhao [19] | China | male | SAMP8 mice | 8 months |
SAMP8 + sham operation (n = 10) SAMP8 + NSC (n = 10) |
Mice NSC from embryo | Stereotactically transplanted | Hippocampus | 5 × 105 | N | 15 days | ①⑥ |
| Zhang [20] | China | male | APPswe/PS1dE9 mice | 12 months |
Tg + PBS (n = 10) Tg + NSC (n = 10) |
Mice NSC | Stereotactically transplanted | Hippocampus | 5 × 105 to 1 × 106 | N | 10 weeks | ①④ |
| Zhang [21] | China | NA | APPswe/PS1dE9mice | 12 months |
Tg + vehicle (n = 20) Tg + NSC (n = 20) |
Mice NSC from 14 days embryo | Stereotactically transplanted | Hippocampus | 2.5–5 × 106 | N |
5 weeks 10 weeks |
① |
| Mathew [22] | USA | NA | APP/PS1/tau 3 × Tg AD mice | 18 months |
3xTg-AD + vehicle (n = 9) 3xTg-AD + NSC (n = 18) |
Mice NSC (postnatal day 1) | Stereotactically transplanted | Hippocampus | 1 × 105 | N | 1 month | ① |
| Chen [23] | China | Male | APP/PS1/tau 3 × Tg AD mice | 12 months |
3xTg-AD + PBS (n = 10) 3xTg-AD + NSC (n = 10) |
Mice NSC from hippocampus and subependymal zone of 12.5 days fetal brain | Stereotactically transplanted | Hippocampus | 2 × 106 | N | 8 weeks | ① |
| Lu [24] | China | male | APPswe/PS1dE9 mice | 3.5 months |
AD + saline AD + NSC |
Human NSC from hippocampus of 6–8-week embryos | Intranasally transplanted | Nasal cavity | 1 × 106, 4 times | Y |
3 months 4 months |
①②④⑤⑥ |
| Zhang [25] | China | NA | Tg-tau mice | 40 weeks |
Tg + PBS (n = 11) Tg + NSC (n = 11) |
Mice NSC from hippocampus on postnatal day1 |
Stereotactically transplanted |
Hippocampus | 2 × 105 | N | 4 weeks | ①③ |
| Zhang [26] | China | male | APPswe/PS1dE9 mice | 12 months |
AD + PBS (n = 20) AD + NSC (n = 20) |
Mice NSC from embryonic day 14 | Stereotactically transplanted | Hippocampus |
5 × 105to 1 × 106 |
N | 10 weeks | ①②⑤ |
| Zhang [27] | China | NA | APPswe/PS1dE9 mice | 10 months |
AD + Vehicle (n = 15) AD + NSC (n = 15) |
Mice NSC from embryonic day 14 | Stereotactically transplanted | Hippocampus | 1 × 106 | N | 8 weeks | ①④ |
| Zhang [28] | China | NA | APPswe/PS1dE9 mice | 12 months |
AD + Vehicle (n = 20) AD + NSC (n = 20) |
Mice NSC from embryonic day 14 | Stereotactically transplanted | Hippocampus | 5 × 105 to 1 × 106 | N | 8 weeks | ①②④⑥ |
| Zhou [29] | China | male | SAMP8 mice | 8 months |
SAMP8 control (n = 10) SAMP8 + NSC (n = 10) SAMP8 SAMP8 + NSC + HuangDiSan (n = 10) |
Mice NSC from embryonic day 12–16 | Stereotactically transplanted | Hippocampus | 1 × 106 | N | 15 days | ①④ |
| Ofra [30] | Israel | male | APPswe/PS1dE9 mice | 11 months |
AD + sham AD + NPC AD + IL-1ra-NPC |
Mice NPC | Stereotactically transplanted | Hippocampus | 4000 spheres | N | 1 month | ①②⑥ |
| Armijo [31] | USA | male and female | APP/PS1/tau 3 × Tg AD mice | 17 months |
3xTg-AD + PBS 3xTg-AD + NPC |
Mice NPC from tail-tip fibroblasts | Stereotactically transplanted | Hippocampus | 5 × 105 | N |
1 month 2 months |
②③ |
| Lee [32] | Korea | NA | NSE/APPsw transgenic mice | 13 months |
APP + Vehicle APP + NSC |
Human NSC from 13-week fetal brain | ICV | Lateral ventricles | 5 × 105 | Y |
6 weeks 12 weeks |
①②③④⑤⑥ |
| Li [33] | China | male and female | APPswe/PS1dE9 mice | 12 months |
AD + Vehicle (n = 10) AD + NSC (n = 10) |
Mice NSC from embryonic day 14 | Stereotactically transplanted | Hippocampus | 2.5–5 × 106 | N | 3 weeks | ①⑤⑥ |
| Li [34] | China | NA | APPswe/PS1dE9 mice | NA |
AD + Saline AD + NSC AD + CSeM/let-7b NPs-NSC |
Mice NSC from hippocampus | Stereotactically transplanted | NA | NA | N | 30 days | ①⑥ |
| Lilja [35] | Sweden | male and female | Tg2576 Mice | 6–9 months |
Tg + Vehicle + saline (n = 9) Tg + NSC + saline (n = 9) Tg + NSC + JN403 (n = 5) Tg + NSC + ( +)-phenserine (n = 7) |
Human NSC | Stereotactically transplanted | Hippocampus | 2.5 × 104 | N | 5 weeks | ①② |
| Park [36] | Korea | NA | APPswe/PS1dE9 mice | 18 months |
AD + saline (n = 10) AD + NSC (n = 10) AD + CHAT-NSC (n = 10) |
Human NSC from 15-week fetal brain | ICV | Lateral ventricles | 1 × 106 | N | 4 weeks | ①②⑥ |
NA not reported, AD Alzheimer’s disease, NSC neural stem cell, NPC neural progenitor cell, N No, Y Yes, IBO acid ibotenic acid, ICV intra-cerebroventricular injection, ① Morris water maze test, ② Aβ deposition, ③ p-tau level, ④ synaptic density, ⑤ anti-inflammatory effect, ⑥ brain-derived neurotrophic factor level
Table 2.
Characteristics of rat trials
| References | Location | Animal sex | Animal species | Animal weight | Animal year | Group | Type and source of NSPC | Way of administrated | Dose of NSPC | Immunosuppression | Assessment time | Parameter | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Park [37] | Korea | Male | SD rat | 220–230 g | NA |
AF64A (n = 15) AF64A + NSC (n = 15) AF64A + ChAT NSC (n = 15) |
Human NSC from 15-week fetal brain | ICV | Right ventricle | 1 × 106 | N |
4–5 weeks 8–9 weeks |
MWM |
| Moghadam [38] | Iran | Male | SD rat | about 300 g | NA |
nbM lesion + vehicle (n = 6) nbM lesion + NPC (n = 6) |
Mice NPC differentiation from ESC | Stereotactically transplanted | Right nbM | 2 × 105 | Y | 4 weeks | MWM |
| Tang [39] | China | Male | Wistar rat | 200–250 g | 3–4 months |
Aβ (n = 10) Aβ + NPC (n = 10) |
Mice NPC from embryonic fibroblasts | Stereotactically transplanted | Hippocampus | NA | Y |
4 weeks 16 weeks |
MWM |
| Wu [40] | Japan | Male | Wistar rat | 270–290 g | NA |
OA (n = 12) OA + NSC (n = 12) OA + NSC-hNGF-eGFP (n = 12) |
Rat NSC from 17-day rat forebrain cerebral cortex | Stereotactically transplanted | Hippocampus and cerebral cortex | 2 × 105 | N | 30 days | MWM |
| Chen [41] | China | Male | SD rat | 200–250 g | NA |
IgG-saporin (n = 8) IgG-saporin + NSC (n = 8) IgG-saporin + NSC + NGF-PE-PLGA-NPs (N = 8) |
Rat NSC from embryonic day 13.5–15.5 | Stereotactically transplanted | Hippocampus and basal forebrain | NA | N | 4 weeks | MWM |
| Cui [42] | China | Female | SD rats | NA | NA |
Aβ (n = 15) Aβ + NSC (n = 15) Aβ + NSC + DSP (n = 15) |
Rat NSC from hippocampus on postnatal day1 | Stereotactically transplanted | Hippocampus | 5 × 105 | N | 4 weeks | MWM |
| Hu [43] | China | Female | SD rats | NA | 8 weeks |
Aβ (n = 18) Aβ + NSC (n = 18) Aβ + NSC + ASI (n = 18) |
Rat NSC from embryonic day 14 | Stereotactically transplanted | Hippocampus | 1 × 105 | N | 4 weeks | MWM |
| Shaymaa [44] | Egypt | Male | SD rats | 200–250 g | 3 months |
IBO acid (n = 10) IBO acid + NSC (n = 10) IBO acid + NSC + ROO (n = 10) |
Human adult OBNSCs | Stereotactically transplanted | Hippocampus | 2.4 × 105 | Y | 7 weeks | MWM |
NA not reported, SD rat Sprague Dawley rat, OA okadaic acid, IBO acid Ibotenic acid, NSC neural stem cell, NPC neural progenitor cell, N no, Y yes, OBNSCs olfactory bulb neuronal stem cells, DSP designer self-assemble peptide, ROO rosemary oil, MWM Morris water maze, ICV intra-cerebroventricular injection, nbM nucleus basalis of Meynert
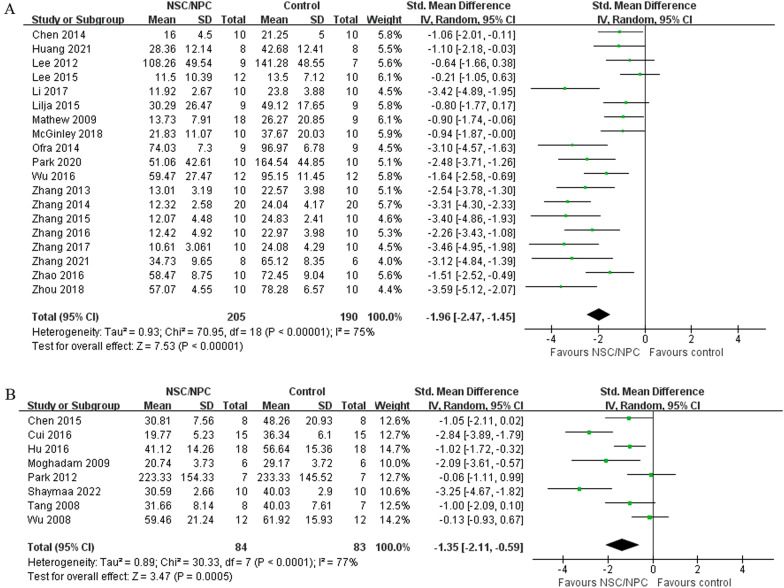
Cognitive function
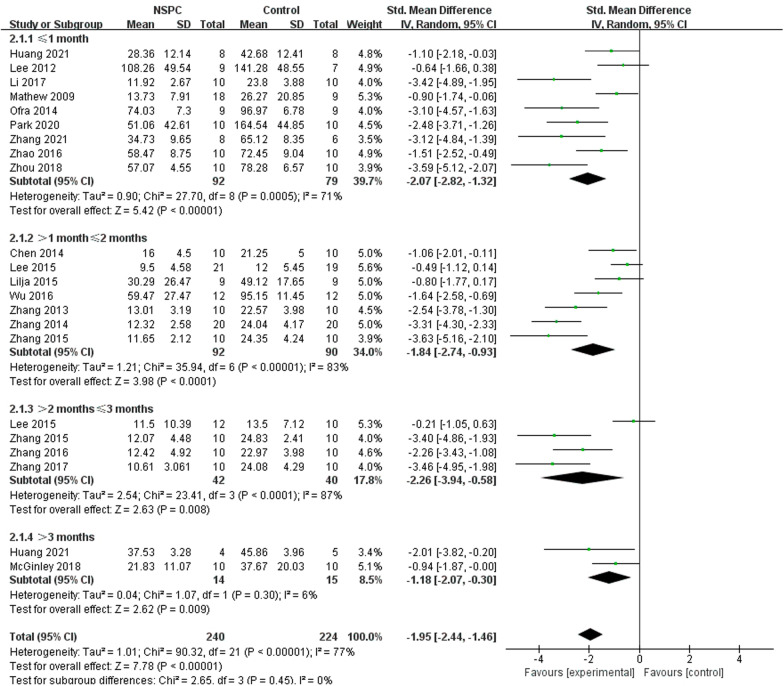
Cognitive function was assessed by Morris water maze (MWM), and we extracted the data of escape latency from the last day of the learning phase. Nineteen of mice studies [15–23, 25–30, 32, 33, 35, 36] included MWM testing, we used a random-effect model to compare NSPC group (205 mice) and control group (190 mice), and the analysis showed that compared with the control group, NSPC could improve cognitive function apparently (SMD = − 1.96, 95% CI − 2.47 to − 1.45, I2 = 75%, P < 0.00001) (Fig. 3a). Eight rat studies [37–44] included MWM texting, we also used a random-effect model, and the outcome showed that cognitive function compared with control group (83 rats) and NSPC group (84 rats) improved apparently (SMD = − 1.35, 95% CI − 2.11 to − 0.59, I2 = 77%, P = 0.0005)(Fig. 3b).
Fig. 3.
Forest plot shows the mean effect size and 95% confidence interval (CI) for cognitive function of mice studies A and rat studies B between NSPC treatment group and control group
Pathological features
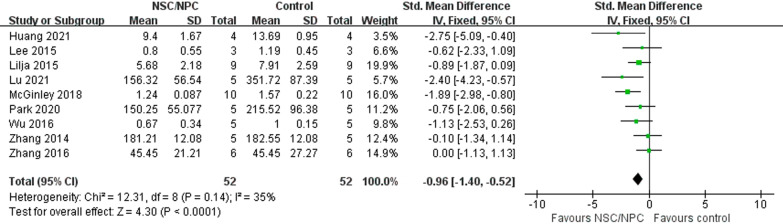
Aβ deposition
In mice studies, 9 studies [15, 16, 18, 24, 26, 28, 32, 35, 36] reported the difference of NSPC group (52 mice) and control group (52 mice) about Aβ deposition. We used a fixed-effect model for low heterogeneity (P = 0.14, I2 = 35%). Meta-analyses showed that Aβ deposition after NSPC treatment was significantly lower than AD models (SMD = − 0.96, 95% CI − 1.40 to − 0.52, P < 0.0001) (Fig. 4).
Fig. 4.
Forest plot shows the mean effect size and 95% confidence interval (CI) for Aβ deposition of mice studies between NSPC treatment group and control group
Synaptic density
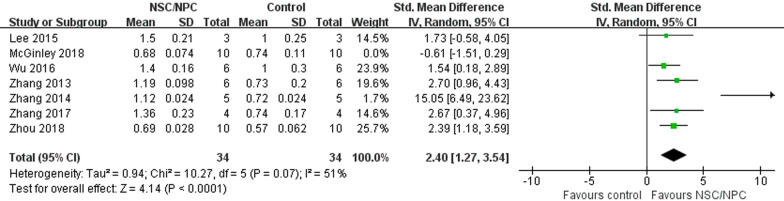
We used synaptophysin (SYP) expression to evaluate synaptic density to ensure if synaptic loss had been ameliorated, and 7 mice studies [16, 18, 20, 27–29, 32] reported it. Because of high heterogeneity (P < 0.00001, I2 = 82%) (Additional files 4: Fig. S2), we used a random-effect model, which showed that SYP expression of NSPC group (44 mice) was significantly higher than control group (44 mice), suggesting that NSPC promotes synaptic density recovery (SMD = 2.02, 95% CI 0.50–3.55, P = 0.009). Sensitivity analysis showed that high heterogeneity could be explained by the work of McGinley et al. [18]. After it was excluded, the level of heterogeneity decreased (P = 0.07, I2 = 51%) (Fig. 5).
Fig. 5.
Forest plot shows the mean effect size and 95% confidence interval (CI) for synaptic density of mice studies between NSPC treatment group and control group
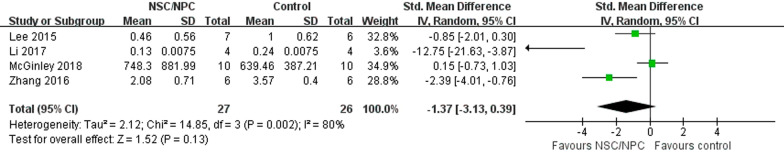
Anti-inflammatory effect
IL-1β expression was used to assess anti-inflammatory effect of NSPC treatment, and 4 mice studies [18, 26, 32, 33] reported it. A random-effects model was used for the analysis because of the high heterogeneity (P = 0.002, I2 = 80%), and the results indicated that IL-1β expression did not change significantly (SMD = − 1.37, 95% CI − 3.13 to 0.39, P = 0.13) (Fig. 6).
Fig. 6.
Forest plot shows the mean effect size and 95% confidence interval (CI) for anti-inflammatory effect of mice studies between NSPC treatment group and control group
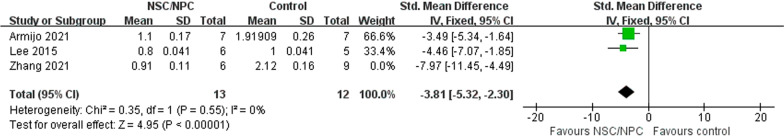
P-tau
A total of 3 mice studies [25, 31, 32] compared p-tau level between NSPC group (19 mice) and control group (21 mice), and we used a random-effects model for the analysis because of the high heterogeneity (P = 0.08, I2 = 60%). The outcome showed that the p-tau level of NSPC group is lower (SMD = − 4.94, 95% CI − 7.29 to − 2.59, P < 0.0001) (Additional files 5: Fig. S3). Sensitivity analysis showed that high heterogeneity could be explained by the work of Zhang et al. [25]. After it was excluded, the level of heterogeneity decreased (P = 0.55, I2 = 0%) (Fig. 7). But due to the small number of data, we need more studies to make a conclusion.
Fig. 7.
Forest plot shows the mean effect size and 95% confidence interval (CI) for p-tau level of mice studies between NSPC treatment group and control group
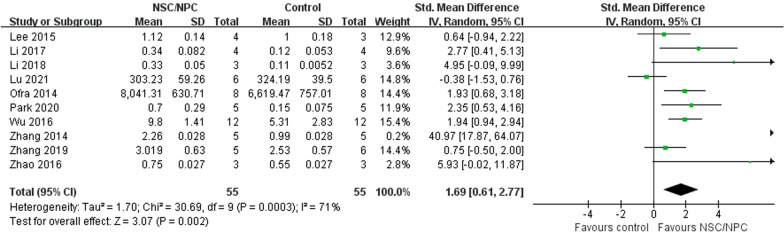
Brain-derived neurotrophic factor (BDNF)
A total of 10 mice studies [16, 19, 24, 28, 30, 32–34, 36, 45] reported BDNF level, we used a random-effect model to compare BDNF level between NSPC group (55 mice) and control group (55 mice) because of high heterogeneity (P = 0.0003, I2 = 71%). BDNF level of NSPC group was higher than control group (SMD = 1.69, 95% CI 0.61–2.77, P = 0.002) (Fig. 8).
Fig. 8.
Forest plot shows the mean effect size and 95% confidence interval (CI) for BDNF level of mice studies between NSPC treatment group and control group
Effect of NSPC combined with other treatment
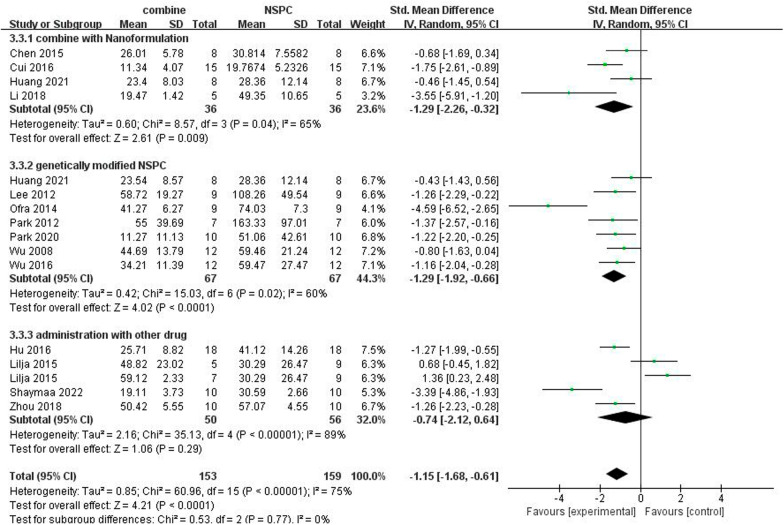
A total of 14 studies used NSPC combined with other treatment, we divided them into 3 group: a: NSPC combined with nanoformulation (4 studies) [15, 33, 41, 42], b: genetically modified NSPC (7 studies) [15–17, 30, 36, 37, 40], and c: NSPC administration with other drug (4 studies with 5 drugs) [29, 35, 43, 44]. We used a subgroup analysis to compare the effect between combination group with NSPC group on cognitive function (Fig. 9). The outcome proved that both combined with nanoformulation (SMD = − 1.29, 95% CI − 2.26 to − 0.32, I2 = 65%, P = 0.009) and genetically modified NSPC (SMD = − 1.29, 95% CI − 1.92 to − 0.66, I2 = 60%, P < 0.0001) can enhance the effect of NSPC therapy. But consolidated analysis suggested that there was no statistically significant difference in cognitive function between NSPC treatment and NSPC administration with other drug (SMD = − 0.74, 95% CI − 2.12 to 0.64, I2 = 89%, P = 0.29).
Fig. 9.
Forest plots of subgroup analysis by effect of NSPC combined with other treatment for cognitive function in preclinical rodent models
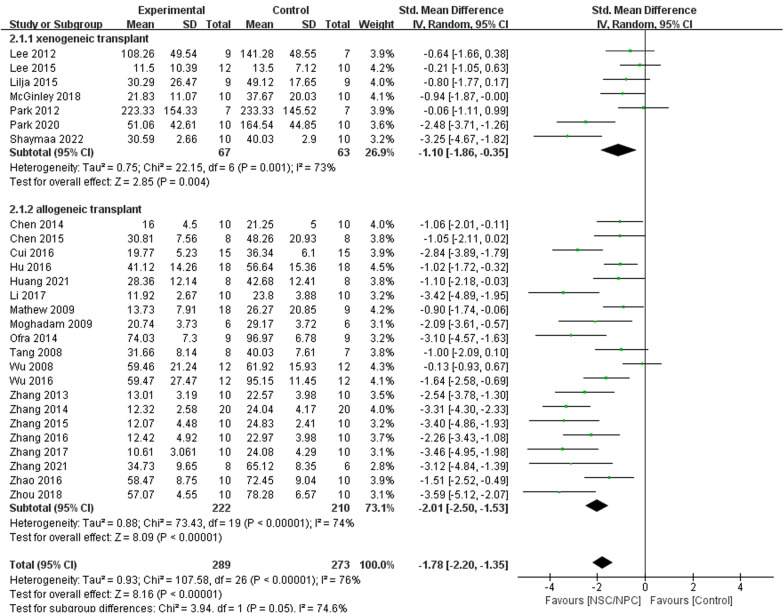
Effect of NSPC xenogeneic and allogeneic transplant for cognitive function
Nineteen of mice studies and 8 rat studies included MWM testing, and we divided them into 2 groups: xenogeneic transplant group (7 studies) [17, 18, 32, 35–37, 44] and allogeneic transplant group (20 studies) [15, 16, 19–23, 25–30, 33, 38–43]. We used a subgroup analysis to evaluate the effect of NSPC xenogeneic transplant and allogeneic transplant on cognitive function (Fig. 12). The outcome proved that both xenogeneic transplant (SMD = − 1.10, 95% CI − 1.86 to − 0.35, I2 = 73%, P = 0.004) and allogeneic transplant (SMD = − 2.01, 95% CI − 2.50 to − 1.53, I2 = 74%, P < 0.00001) treatment could improve cognitive function apparently.
Fig. 12.
Forest plots of subgroup analysis by assessment time for cognitive function in rat model
Sensitivity analysis
To evaluate the stability of the results, we further performed a sensitivity analysis through the sequential omission of each study. For the pooled SMD, outcome of cognitive function, Aβ deposition and BDNF level were not significantly affected by any study.
Discussion
Current treatments of AD are unable to achieve satisfactory therapeutic outcomes, so an effective and safe treatment is urgently required. We explored whether NSPC could be used to treat AD. Our meta-analysis of 30 studies made a comprehensive summary about the effect of NSPC therapy on the mice and rat model of AD. Pooled analyses confirmed that NSPC therapy could improve cognitive function in the preclinical models of AD. Our analysis also suggests that inject NSPC with nanoformulation and genetically modified boost the efficacy of NSPC treatment. Therefore, the present meta-analysis provides significant clues for human clinical trials on NSPC therapy.
Alzheimer’s disease is a progressive neurodegenerative disorder, which is a major cause of dementia [46], so we chose cognitive function as outcome indicate. The pathological features of AD include the presence of extracellular Aβ-containing senile plaques and intracellular hyperphosphorylated tau-containing NFT, neuroinflammation and synaptic loss, so we used Aβ deposition, synaptic density, anti-inflammatory effect and p-tau level as pathological indication. We found that BDNF was observed in several studies, so we analyzed the change of BDNF to evaluate the function of NSPC therapy.
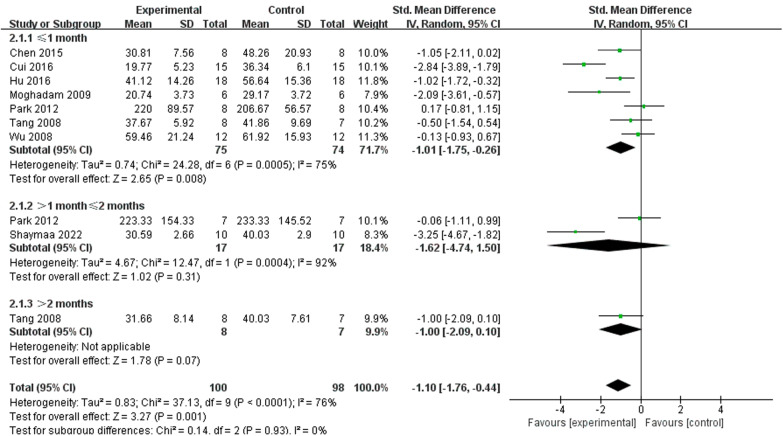
Morris water maze (MWM) experiment is widely used in scientific research to assess the learning and memory of animals [47]. Almost all studies use MWM experiment as behavioral experiments to observe whether cognitive function has improved. In this analysis, we used the data of escape latency from the last day of the learning phase to evaluate the cognitive function. Compared to control group, almost all data of NSPC treatment group were lower, which means that NSPC therapy could improve the learning and memory function of AD model and ameliorate the deterioration of cognitive function. The subgroup analysis of assessment time in mice trials showed that after 3 months, NSPC therapy still has effectiveness (SMD = − 1.18, 95% CI − 2.07 to 0.30, I2 = 6%, P = 0.009) (Fig. 10). But in rat studies, after 1 month, NSPC does not work (SMD = − 1.62, 95% CI − 4.74 to 1.50, I2 = 92%, P = 0.31) (Fig. 11).
Fig. 10.
Forest plots of subgroup analysis by effect of NSPC allogeneic transplant and NSPC xenogeneic transplant for cognitive function in preclinical rodent models
Fig. 11.
Forest plots of subgroup analysis by assessment time for cognitive function in mice model
Aβ is one of the key initiating factors of AD pathogenesis. Accumulation of Aβ results in loss of synapses, neuroinflammation and ultimately cognitive deficits [48]. Our analysis collected the data about Aβ expression of NSPC group and control group; compared to control group, Aβ deposition of NSPC group was significantly lower, so we can conclude that NSPC decreases Aβ accumulation. Tau proteins are microtubular neuronal proteins. The tau proteins have a microtubule binding domain, which is involved in polymerization and stabilization of the microtubule assembly to maintain the integrity of the cytoskeleton. Hyperphosphorylation results in decreased affinity of the tau proteins to microtubules. The hyperphosphorylated tau forms NFTs and gets deposited in the cytosol and can no longer perform the function of maintaining the structure of the cell [46]. Moreover, it would impair cognitive function. Of all studies, 3 studies [25, 31, 32] reported p-tau level and suggested that NSPC treatment would reduce p-tau aggregation (Fig. 12).
A synaptic damage in the neocortex and limbic system causes memory impairment and generally is observed at the early stages of AD [49]. SYP is a specific protein on the membrane of synaptic vesicles, which may be involved in the formation of synaptic vesicles and dendrite spine. Here, we used SYP to evaluate synaptic density. The data of NSPC group were higher than control group, and it can prove that NSPC transplantation enhances synaptic density, attenuated the synaptotoxic properties of Aβ and promoted synaptic plasticity [32]. Electrophysiological recording of 2 studies [31, 45] also proved that NSPC transplantation promoted synaptic plasticity.
Many studies now point to the involvement of neuroinflammation playing a fundamental role in the progression of the neuropathological changes that are observed in AD [50]. Unlike other risk factors and genetic causes of AD, neuroinflammation is not typically thought to be causal on its own but rather a result of one or more of the other AD pathologies or risk factors associated with AD and serves to increase the severity of the disease by exacerbating β-amyloid and tau pathologies [51, 52]. IL-1β has been described as a “master regulator” within the brain inflammatory cascade, and disruptions to IL-1β can delay the onset of neuroinflammation and neurodegeneration [53]. We used IL-1β expression to evaluate neuroinflammation, though the data we collected of NSPC group were lower than control group, and there was no statistical significance between two groups. One study quantified the density of microglia and astrocytes and proved that NSCs transplantation reduced the density of astrocytes and microglia, suggesting that NSCs inhibit neuroinflammation [24, 54].
In the brain, BDNF is expressed by glutamatergic neurons and glial cells, such as astrocytes isolated from the cortex and hippocampus [54, 55]. BDNF is a neurotrophin that modulates the survival of stem cells and progenitors, neurogenesis and neuronal differentiation, the branching and survival of differentiated neurons and the formation and maturation of the dendritic spine and synapses. Thus, BDNF influences learning and memory [56]. And our analysis demonstrated that NSPC treatment could improve BDNF level to ameliorate the condition of AD.
Limitations
Several potential limitations of our meta-analysis should be considered. First, although we performed stratified and sensitivity analyses, the heterogeneity among studies could not be remarkably reduced. This may influence the stability of the results. Second, data of Aβ deposition, SYP expression, tau level and more indicators were lacked in several studies, and role of NSPC in AD alleviation requires further evaluation. Third, our meta-analysis only observed mice and rat models, which are not able to well simulate the physical conditions of human suffered from AD.
Conclusion
The data of our meta-analysis revealed, NSPC transplantation may enhance the cognitive function and reduce AD burden, while the nanoformulation and genetically modification may promote the effect of NSPC therapy. Which would provide the theoretical foundation and guide for clinical trials of NSPC for AD. Both xenogeneic and allogeneic transplant of NSPC could improve the cognitive function of AD animals. More animal studies and human trials are needed for further investigation.
Supplementary Information
Additional file 1: Table S1. The detailed search strategy.
Additional file 2: Fig. S1. Evaluation of publication bias. Funnel plots for Aβ deposition (A), mice cognitive function (B), rat cognitive function (C) and BDNF (D).
Additional file 3: Table S2. SYRCLE’s RoB tool for each experimental animal studies.
Additional file 4: Fig. S2. Forest plot for synaptic density of mice studies between NSPC treatment group and control group. It had high heterogeneity before the work of McGinley et al. was excluded.
Additional file 5: Fig. S3. Forest plot for p-tau level of mice studies between NSPC treatment group and control group. Because of high heterogeneity, we used a random-effect model.
Acknowledgements
This work was supported by Hebei Medical University and funded by Natural Science Foundation of China (81801278), China Scholarship Council (201608130015), Natural Science Foundation of Hebei Province (H2019206637), Key Natural Science Foundation of Hebei Province (H2020206557), Overseas researcher Program in Hebei Provincial Department of human resources and social security (C20190509) and Natural Science Foundation of Hebei Province (H2015206409).
Abbreviations
- AD
Alzheimer’s disease
- NSPC
Neural stem/progenitor cell
- SMD
Standard mean difference
- CI
Confidence interval
- Aβ
Amyloid-β
- NFT
Neurofibrillary tangles
- FAD
Familial Alzheimer disease
- SAD
Sporadic Alzheimer disease
- AChEIs
Acetylcholinesterase inhibitors
- NMDAR
N-Methyl-d-aspartate receptor
- DBS
Deep brain stimulation
- rTMS
Repetitive transcranial magnetic stimulation
- MSC
Mesenchymal stem cell
- iPSC
Induced pluripotent stem cell
- NSC
Neural stem cell
- ESC
Embryonic stem cell
- MeSH
Medical subject headings
- SD rat
Sprague Dawley rat
- OA
Okadaic acid
- IBO acid
Ibotenic acid
- OBNSCs
Olfactory bulb neuronal stem cells
- DSP
Designer self-assemble peptide
- ROO
Rosemary oil
- MWM
Morris water maze
- SYP
Synaptophysin
- BDNF
Brain-derived neurotrophic factor
- ICV
Intra-cerebroventricular injection
- nbM
Nucleus basalis of Meynert
Author contributions
JM and HXC conceived and designed the study. ZJZ, BS and YXX selected the articles and extracted and cross-checked the data. ZJZ, BS, JYZ, XL, XHZ and BFF contributed to the statistical analysis. ZJZ wrote the first draft of the manuscript. ZJZ, BS and JM revised and discussed the final edition. All authors read and approved the final manuscript.
Funding
This study was funded by Natural Science Foundation of China (81801278), China Scholarship Council (201608130015), Natural Science Foundation of Hebei Province (H2019206637), Key Natural Science Foundation of Hebei Province (H2020206557), Overseas researcher Program in Hebei Provincial Department of human resources and social security (C20190509) and Natural Science Foundation of Hebei Province (H2015206409).
Availability of data and materials
All supporting data are included in the article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zijing Zhou and Ben Shi contribute equally
References
- 1.Larson EB, Kukull WA, Katzman RL. Cognitive impairment: dementia and Alzheimer's disease. Annu Rev Public Health. 1992;13(1):431–449. doi: 10.1146/annurev.pu.13.050192.002243. [DOI] [PubMed] [Google Scholar]
- 2.Yankner BA, Lu T. Amyloid β-protein toxicity and the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284(8):4755–4759. doi: 10.1074/jbc.R800018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masters CL, et al. Alzheimer's disease. Nat Rev Dis Primers. 2015;1:15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe MS. In search of pathogenic amyloid β-peptide in familial Alzheimer's disease. Prog Mol Biol Transl Sci. 2019;168:71–78. doi: 10.1016/bs.pmbts.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju Y, Tam KY. Pathological mechanisms and therapeutic strategies for Alzheimer's disease. Neural Regen Res. 2022;17(3):543–549. doi: 10.4103/1673-5374.320970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kevadiya BD, et al. Neurotheranostics as personalized medicines. Adv Drug Deliv Rev. 2019;148:252–289. doi: 10.1016/j.addr.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponce FA, et al. Bilateral deep brain stimulation of the fornix for Alzheimer's disease: surgical safety in the ADvance trial. J Neurosurg. 2016;125(1):75–84. doi: 10.3171/2015.6.JNS15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koch G, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer's disease. Neuroimage. 2018;169:302–311. doi: 10.1016/j.neuroimage.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 9.Martorell AJ, et al. Multi-sensory Gamma stimulation ameliorates Alzheimer's-associated pathology and improves cognition. Cell. 2019;177(2):256–271.e22. doi: 10.1016/j.cell.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakthiswary R, Raymond AA. Stem cell therapy in neurodegenerative diseases: from principles to practice. Neural Regen Res. 2012;7(23):1822–1831. doi: 10.3969/j.issn.1673-5374.2012.23.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: a phase I clinical trial. Alzheimers Res Ther. 2021;13(1):154. doi: 10.1186/s13195-021-00897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brody M, et al. Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer's disease. Alzheimers Dement. 2022 doi: 10.1002/alz.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tincer G, et al. Neural stem/progenitor cells in Alzheimer's disease. Yale J Biol Med. 2016;89(1):23–35. [PMC free article] [PubMed] [Google Scholar]
- 14.Hooijmans CR, et al. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang D, et al. A nanoformulation-mediated multifunctional stem cell therapy with improved beta-amyloid clearance and neural regeneration for Alzheimer's disease. Adv Mater. 2021;33:13. doi: 10.1002/adma.202006357. [DOI] [PubMed] [Google Scholar]
- 16.Wu CC, et al. Gain of BDNF function in engrafted neural stem cells promotes the therapeutic potential for Alzheimer's disease. Sci Rep. 2016;6:27358. doi: 10.1038/srep27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HJ, et al. Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant. 2012;21(11):2487–2496. doi: 10.3727/096368912X638964. [DOI] [PubMed] [Google Scholar]
- 18.McGinley LM, et al. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer's disease. Sci Rep. 2018;8:1–10. doi: 10.1038/s41598-018-33017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao L, et al. Acupuncture improves cerebral microenvironment in mice with Alzheimer's disease treated with hippocampal neural stem cells. Mol Neurobiol. 2017;54(7):5120–5130. doi: 10.1007/s12035-016-0054-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, et al. NSCs promote hippocampal neurogenesis, metabolic changes and synaptogenesis in APP/PS1 transgenic mice. Hippocampus. 2017;27(12):1250–1263. doi: 10.1002/hipo.22794. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, et al. Neural stem cell transplantation enhances mitochondrial biogenesis in a transgenic mouse model of Alzheimer's disease-like pathology. Neurobiol Aging. 2015;36(3):1282–1292. doi: 10.1016/j.neurobiolaging.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Blurton-Jones M, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106(32):13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, et al. Neural stem cell transplantation improves spatial learning and memory via neuronal regeneration in amyloid-beta precursor protein/presenilin 1/tau triple transgenic mice. Am J Alzheimers Dis Other Dement. 2014;29(2):142–149. doi: 10.1177/1533317513506776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu MH, et al. Intranasal transplantation of human neural stem cells ameliorates Alzheimer's disease-like pathology in a mouse model. Front Aging Neurosci. 2021;13:650103. doi: 10.3389/fnagi.2021.650103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang HA, et al. Neural stem cell transplantation alleviates functional cognitive deficits in a mouse model of tauopathy. Neural Regen Res. 2022;17(1):152–162. doi: 10.4103/1673-5374.314324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, et al. Neural stem cell transplantation decreases neuroinflammation in a transgenic mouse model of Alzheimer's disease. J Neurochem. 2016;136(4):815–825. doi: 10.1111/jnc.13413. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, et al. Effects of neural stem cells on synaptic proteins and memory in a mouse model of Alzheimer's disease. J Neurosci Res. 2014;92(2):185–194. doi: 10.1002/jnr.23299. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, et al. Neural stem cell transplants improve cognitive function without altering amyloid pathology in an APP/PS1 double transgenic model of Alzheimer's disease. Mol Neurobiol. 2014;50(2):423–437. doi: 10.1007/s12035-014-8640-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou CL, et al. Combined acupuncture and HuangDiSan treatment affects behavior and synaptophysin levels in the hippocampus of senescence-accelerated mouse prone 8 after neural stem cell transplantation. Neural Regen Res. 2018;13(3):541–548. doi: 10.4103/1673-5374.228760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben Menachem-Zidon O, et al. Intra-hippocampal transplantation of neural precursor cells with transgenic over-expression of IL-1 receptor antagonist rescues memory and neurogenesis impairments in an Alzheimer's disease model. Neuropsychopharmacology. 2014;39(2):401–414. doi: 10.1038/npp.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armijo E, et al. Induced pluripotent stem cell-derived neural precursors improve memory, synaptic and pathological abnormalities in a mouse model of Alzheimer's disease. Cells. 2021;10(7):1802. doi: 10.3390/cells10071802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee IS, et al. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol Neurodegener. 2015;10:38. doi: 10.1186/s13024-015-0035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li B, et al. Regulation and effects of neurotrophic factors after neural stem cell transplantation in a transgenic mouse model of Alzheimer disease. J Neurosci Res. 2018;96(5):828–840. doi: 10.1002/jnr.24187. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, et al. Positively charged polyprodrug amphiphiles with enhanced drug loading and reactive oxygen species-responsive release ability for traceable synergistic therapy. J Am Chem Soc. 2018;140(11):4164–4171. doi: 10.1021/jacs.8b01641. [DOI] [PubMed] [Google Scholar]
- 35.Lilja AM, et al. Neural stem cell transplant-induced effect on neurogenesis and cognition in Alzheimer Tg2576 mice is inhibited by concomitant treatment with amyloid-lowering or cholinergic α7 nicotinic receptor drugs. Neural Plast. 2015;2015:370432. doi: 10.1155/2015/370432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park D, et al. Human neural stem cells encoding ChAT gene restore cognitive function via acetylcholine synthesis, a beta elimination, and neuroregeneration in APPswe/PS1dE9 mice. Int J Mol Sci. 2020;21(11):3958. doi: 10.3390/ijms21113958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park D, et al. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp Neurol. 2012;234(2):521–526. doi: 10.1016/j.expneurol.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 38.Moghadam FH, et al. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78(2–3):59–68. doi: 10.1016/j.diff.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Tang J, et al. Embryonic stem cell-derived neural precursor cells improve memory dysfunction in A beta (1–40) injured rats. Neurosci Res. 2008;62(2):86–96. doi: 10.1016/j.neures.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Wu S, et al. Neural stem cells improve learning and memory in rats with Alzheimer's disease. Pathobiology. 2008;75(3):186–194. doi: 10.1159/000124979. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, et al. Treatment efficacy of NGF nanoparticles combining neural stem cell transplantation on Alzheimer's disease model rats. Med Sci Monit. 2015;21:3608–3615. doi: 10.12659/MSM.894567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cui G, et al. Designer self-assemble peptides maximize the therapeutic benefits of neural stem cell transplantation for Alzheimer's disease via enhancing neuron differentiation and paracrine action. Mol Neurobiol. 2016;53(2):1108–1123. doi: 10.1007/s12035-014-9069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haiyan H, et al. Effect of astragaloside IV on neural stem cell transplantation in Alzheimer's disease rat models. Evid Based Complement Alternat Med. 2016;2016:3106980. doi: 10.1155/2016/3106980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezk S, et al. Effects of rosemary oil (rosmarinus officinalis) supplementation on the fate of the transplanted human olfactory bulb neural stem cells against ibotenic acid-induced neurotoxicity (Alzheimer model) in rat. Metab Brain Dis. 2022;37(4):973–988. doi: 10.1007/s11011-021-00890-6. [DOI] [PubMed] [Google Scholar]
- 45.Zhang T, et al. Human neural stem cells reinforce hippocampal synaptic network and rescue cognitive deficits in a mouse model of Alzheimer's disease. Stem Cell Rep. 2019;13(6):1022–1037. doi: 10.1016/j.stemcr.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan S, Barve KH, Kumar MS. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer's disease. Curr Neuropharmacol. 2020;18(11):1106–1125. doi: 10.2174/1570159X18666200528142429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian H, et al. Analysis of learning and memory ability in an Alzheimer's disease mouse model using the morris water maze. J Vis Exp. 2019 doi: 10.3791/60055. [DOI] [PubMed] [Google Scholar]
- 48.Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: time, space and 'wingmen'. Nat Neurosci. 2015;18(6):800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Overk CR, Masliah E. Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochem Pharmacol. 2014;88(4):508–516. doi: 10.1016/j.bcp.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinney JW, et al. Inflammation as a central mechanism in Alzheimer's disease. Alzheimer's Dement Transl Res Clin Interv. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGeer PL, Rogers J. Anti-inflammatory agents as a therapeutic approach to Alzheimer's disease. Neurology. 1992;42(2):447–449. doi: 10.1212/WNL.42.2.447. [DOI] [PubMed] [Google Scholar]
- 52.Zotova E, et al. Inflammation in Alzheimer's disease: relevance to pathogenesis and therapy. Alzheimers Res Ther. 2010;2(1):1. doi: 10.1186/alzrt24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78(2):151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 54.Clarke LE, et al. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018;115(8):E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andreska T, et al. High abundance of BDNF within glutamatergic presynapses of cultured hippocampal neurons. Front Cell Neurosci. 2014;8:107. doi: 10.3389/fncel.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10(3):209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The detailed search strategy.
Additional file 2: Fig. S1. Evaluation of publication bias. Funnel plots for Aβ deposition (A), mice cognitive function (B), rat cognitive function (C) and BDNF (D).
Additional file 3: Table S2. SYRCLE’s RoB tool for each experimental animal studies.
Additional file 4: Fig. S2. Forest plot for synaptic density of mice studies between NSPC treatment group and control group. It had high heterogeneity before the work of McGinley et al. was excluded.
Additional file 5: Fig. S3. Forest plot for p-tau level of mice studies between NSPC treatment group and control group. Because of high heterogeneity, we used a random-effect model.
Data Availability Statement
All supporting data are included in the article.