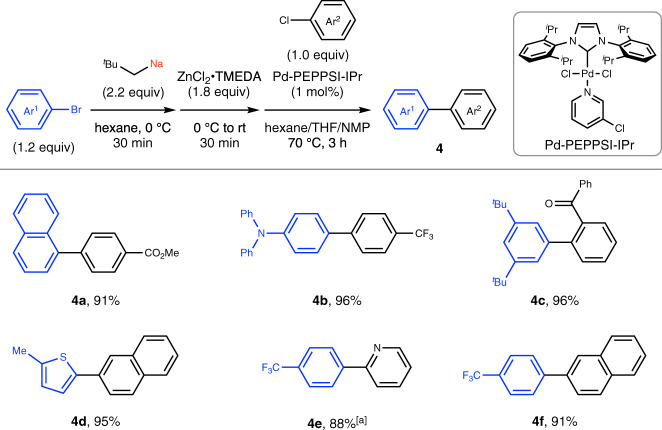
Fig. 5. Palladium-catalyzed Negishi cross-coupling reactions using arylsodiums.
Conditions: step 1, neopentyl chloride (220 mol%) and SD (450 mol%) in hexane (2.0 mL) at 0 °C, 20 min; step 2, Ar1Br (1.2 equiv) was added at 0 °C, 30 min; step 3, ZnCl2•TMEDA (1.8 equiv) was added at 0 °C, stirred at RT for 30 min; step 4, Pd-PEPPSI-IPr (1 mol%), Ar2Cl (0.30 mmol, 1.0 equiv), THF (0.80 mL), and N-methylpyrrolidone (NMP, 0.40 mL) were added and the cross-coupling reaction was performed at 70 °C for 3 h. Yields determined by isolation are shown. aZnCl2•TMEDA (2.2 equiv). SD sodium dispersion, Ar aryl, tBu tert-butyl, TMEDA N,N,N’,N’-tetramethylethylenediamine, THF tetrahydrofuran, NMP N-methyl-2-pyrrolidone, iPr isopropyl, Me methyl, Ph phenyl.