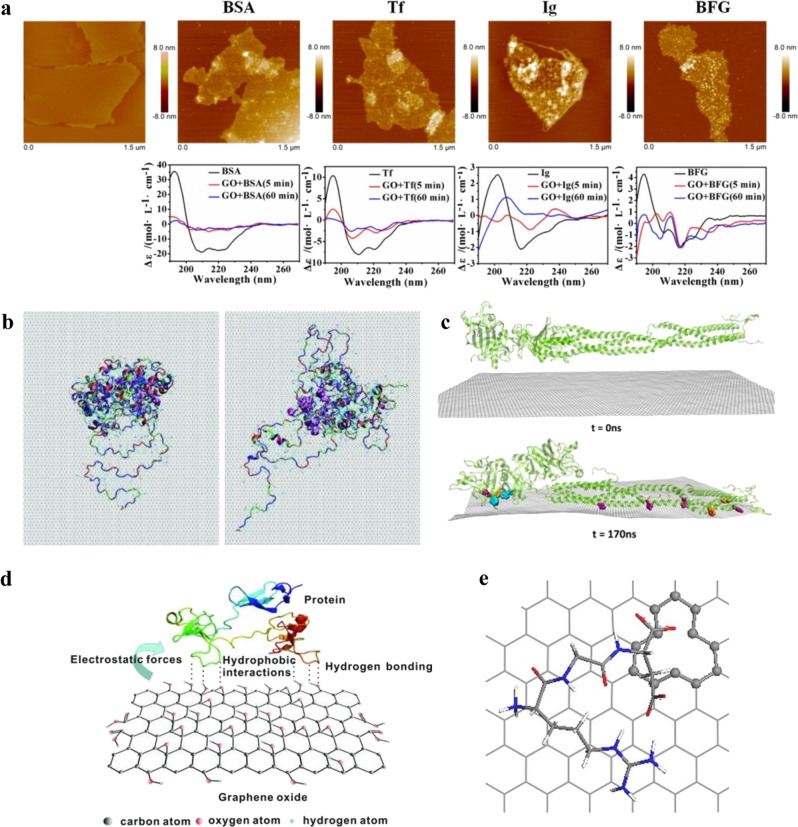
Fig. 1. Multiple interactions between different serum proteins and graphene-based substrates mediate protein binding and conformation.
a Interactions between serum proteins BSA, Tf, IgG, and BFG, with GO along with the corresponding CD spectra, highlighting structural change with incubation time. CD spectra showed that BSA and Tf after adsorption on GO surface exhibited structural rearrangement from α-helical to enhanced β-sheet characteristics, whereas BFG and Ig showed structural heterogeneity on the GO surface (adapted with permission from ref. 29. © 2015 American Chemical Society). b Simulation snapshot of BSA molecule after the 20-ns adsorption showing conformational changes with a decrease in α-helical content on a graphite surface (adapted with permission from ref. 31. © 2011 American Chemical Society). c MD-simulated structural rearrangements of BFG on graphene for 170 ns. Protein aromatic residues Trp, Tyr, and Phe (highlighted in color) oriented and aligned with the graphene surface facilitate π–π stacking (adapted with permission from ref. 29. © 2015 American Chemical Society). d Different interactions of serum proteins on partial oxidized graphene (adapted with permission from ref. 32. © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim). e RGD is attracted to vacancy-defect graphene surfaces with mono-vacancy showing attraction to COO–. The vacancy is highlighted in ball-and-stick style (adapted with permission from ref. 34. © 2015 American Chemical Society).