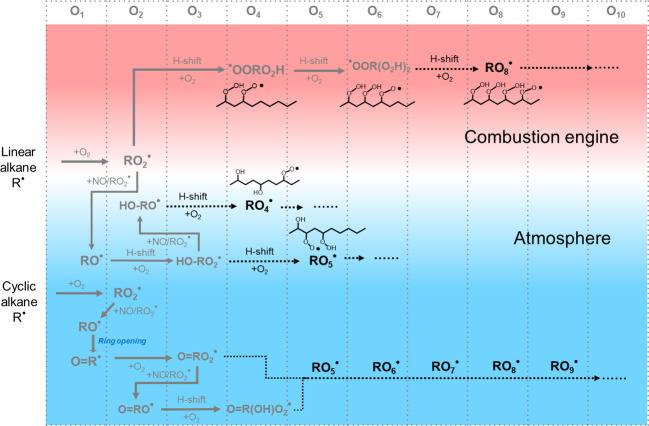
Fig. 1. General reaction mechanisms of peroxy radicals, RO2, from alkane oxidation at combustion-relevant (T > 500 K, red region) and atmospheric (T ≈ 300 K, blue region) conditions.
The figure focuses on possible radical propagation pathways, omitting termination reactions. For linear alkanes, n-decane is used to illustrate some hypothetical example structures. Gray colors depict previously reported radical reactions and intermediates6,18, while the black summarize the results of this study, showing highly oxygenated compounds identified for the first time. The vertical grids separate molecules with different O-atom content. The notations “ROX>2” refer to peroxy radicals with a total of X O-atoms.