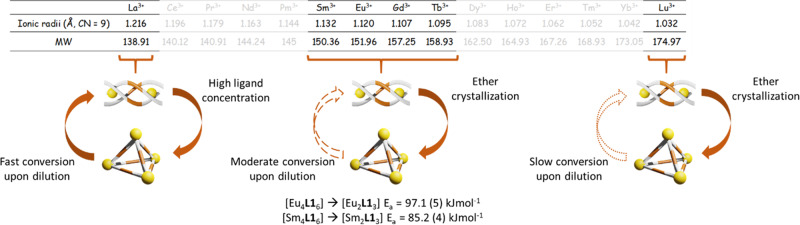
Fig. 6. Summary of helicate-to-tetrahedron transformation by different lanthanide ions.
Ionic radii showed a great influence on the interconvertion between helicate and tetrahedron. Largest La3+ ion prefers to form stable helicate while smallest Lu3+ ions can form both stable helicate and relatively stable tetrahedron assemblies. Lanthanide ions that lies between La and Lu were found to form stable helicates and upon dilution tetrahedron-to-helicate transformation was observed. Both [Eu4L16] and [Sm4L16] exhibited similar activation energy under the tetrahedron-to-helicate transformation process.