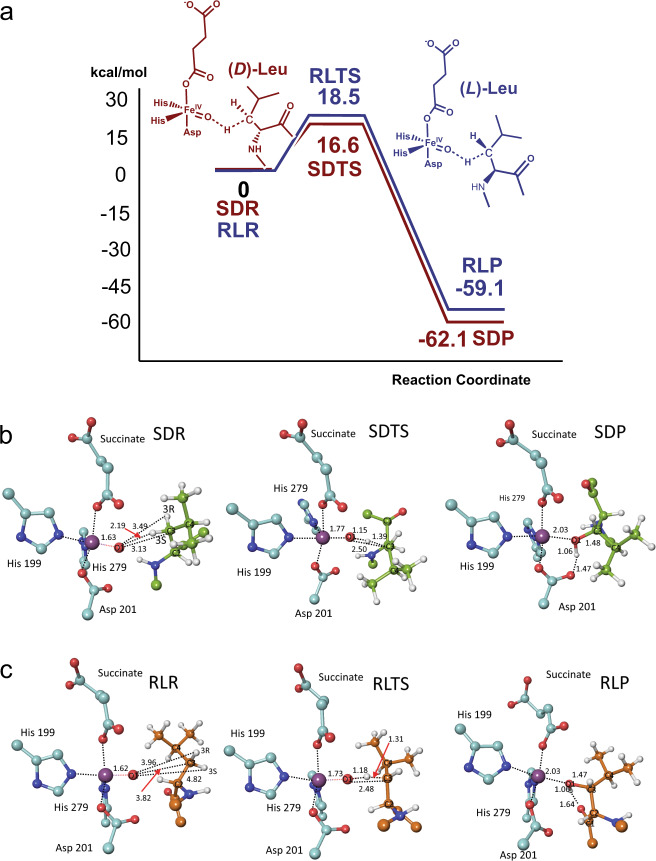
Fig. 3. Mechanistic insights into FIH-catalysed C3 hydroxylation of (D)- and (L)-leucine.
a Potential energy profile diagrams along the modelled reaction coordinates for the FIH-catalysed hydroxylation of (D)-leucine (brown) or (L)-leucine (blue)-derived substrate models. The potential energies of stationary-state structures for the (D)- and (L)-leucine residue substrate hydroxylations are measured from the SDR or RLR intermediate structures, respectively. b Optimized structures of energy minima and transition state for C3 hydroxylation of the (D)-leucine substrate. c Optimized structures of the energy minima and the transition state for C3 hydroxylation of the (L)-leucine substrate. Colours: Fe (blue purple), N (blue), O (red), C (turquoise), substrate (D)-leucine (yellow-green), (L)-leucine (orange), H (white). Selected distances are in Å.