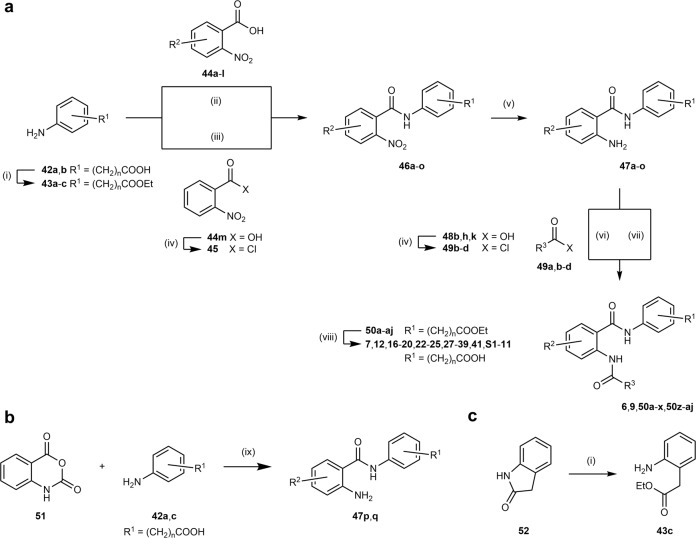
Fig. 2. Synthetic pathways.
Synthesis of 6, 7, 9, 12, 16–20, 22–25, 27–39, 41, S1–11 (a), precursors 47p, q (b), and precursor 43c (c). Reagents and conditions: (i) EtOH, H2SO4, 90 °C, 39–94%. (ii) EDC·HCl, DMAP, CHCl3, 75 °C, 17–93%. (iii) pyridine, THF, 75 °C, 77–99%. (iv) SOCl2, 80 °C (v) H2, Pd/C, EtOAc, rt, or Fe, HOAc, EtOAc, 50 °C or SnCl2, 10% HCl, EtOAc, 50 °C, 33–98%. (vi) pyridine, THF, 75 °C, 35–86%. (vii) R3-COOH (48a,c–g,i,j,l–u), EDC·HCl, DMAP, CHCl3, 75 °C, 20–99%. (viii) (I) LiOH, THF, H2O, rt–50 °C (II) 5% HCl, 20–99%. (ix) EtOH, 90 °C, 74–98%.