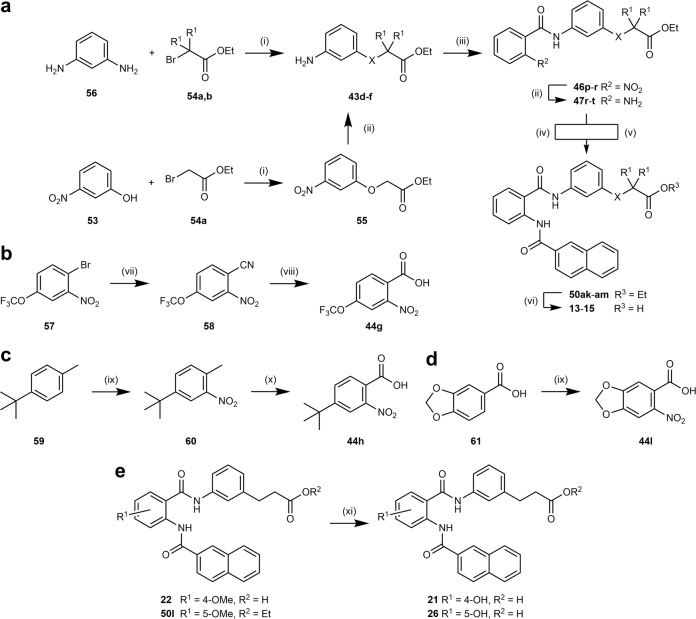
Fig. 3. Synthetic pathways.
Synthesis of 13–15 (a), nitrobenzoic acid precursors 44g (b), 44h (c) and 44l (d), and 21 and 26 (e). Reagents and conditions: (i) K2CO3, DMF, 80 °C, 50–98%. (ii) H2, Pd/C, EtOAc, rt, 64–86%. (iii) 2-nitrobenzoic acid (44 m), EDC·HCl, DMAP, CHCl3, 75 °C, 54–93%. (iv) 2-naphthoic acid (48a), EDC·HCl, DMAP, CHCl3, 75 °C, 65%. (v) 2-naphthoyl chloride (49a), pyridine, THF, 75 °C, 42–45%. (vi) (I) LiOH, THF, H2O, rt – 50 °C (II) 5% HCl, 98–99%. (vii) (I) CuCN, DMF, 150 °C (II) toluene, 130 °C, 92%. (viii) 55% H2SO4 (aq.), 120 °C, 45%. (ix) HNO3, HOAc, Ac2O, 0 °C, 52–76%. (x) KMnO4, pyridine, H2O, 110 °C, 18%. (xi) BBr3, CH2Cl2, 0 °C–rt, 22–65%. X = O, NH; R1 = H, CH3.