Table 1.
Structure–activity relationship investigation of triple FXR/PPARα/δ activators 6–41.
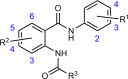 | |||||||
|---|---|---|---|---|---|---|---|
| EC50 [μM] (max. rel. act.) | |||||||
| ID | R1 | R2 | R3 | PPARα | PPARδ | PPARγ | FXR |
| 6 | 2-COOH | — | Naphth-2-yl | tox. | tox. | tox. | 7 ± 1 (44 ± 3%) |
| 7 | 2-CH2COOH | — | Naphth-2-yl | 1.4 ± 0.1 (33 ± 1%) | > 10 | 1.7 ± 0.1 (17 ± 1%) | 9.2 ± 0.2 (31 ± 1%) |
| 8 | 3-COOH | — | Naphth-2-yl | 0.15 ± 0.01 (11 ± 1%) | 1.3 ± 0.1 (14 ± 1%) | 1.6 ± 0.1 (17 ± 1%) | 1.5 ± 0.2 (37 ± 1%) |
| 5 | 3-CH2COOH | — | Naphth-2-yl | 5 ± 1 (37 ± 3%) | 4.9 ± 0.9 (34 ± 2%) | 16 ± 1 (14 ± 1%) | 2.5 ± 0.2 (21 ± 1%) |
| 9 | 3-CH2CH2COOH | — | Naphth-2-yl | 1.0 ± 0.2 (31 ± 2%) | 0.65 ± 0.03 (28 ± 1%) | 2.6 ± 0.3 (19 ± 2%) | 2.6 ± 0.3 (20 ± 1%) |
| 10 | 4-COOH | — | Naphth-2-yl | >30 | >30 | 4.4 ± 0.9 (18 ± 2%) | 1.0 ± 0.2 (23 ± 1%) |
| 11 | 4-CH2COOH | — | Naphth-2-yl | >30 | >30 | >30 | 3.1 ± 0.3 (9.8 ± 0.4%) |
| 12 | 4-CH2CH2COOH | — | Naphth-2-yl | >30 | >30 | >30 | 2.1 ± 0.1 (21 ± 1%) |
| 13 | 3-OCH2COOH | — | Naphth-2-yl | 10 ± 1 (35 ± 2%) | 10 ± 1 (43 ± 4%) | 19 ± 1 (11 ± 1%) | 5 ± 1 (19 ± 1%) |
| 14 | 3-NHCH2COOH | — | Naphth-2-yl | >30 | 3.5 ± 0.2 (83 ± 3%) | 5.7 ± 0.1 (7.7 ± 0.1%) | 1.3 ± 0.1 (31 ± 1%) |
| 15 | 3-NHC(CH3)2COOH | — | Naphth-2-yl | 1.7 ± 0.1 (71 ± 3%) | 3.6 ± 0.2 (42 ± 1%) | 5.1 ± 0.9 (18 ± 1%) | 0.45 ± 0.01 (10 ± 1%) |
| 16 | 3-CH2CH2COOH | 3-CH3 | Naphth-2-yl | 6.2 ± 0.1 (31 ± 1%) | 2.7 ± 0.3 (30 ± 1%) | 7.1 ± 1.3 (6 ± 1%) | i.a. |
| 17 | 3-CH2CH2COOH | 4-CH3 | Naphth-2-yl | 1.7 ± 0.2 (67 ± 3%) | 0.17 ± 0.02 (34 ± 1%) | 1.4 ± 0.3 (21 ± 2%) | 1.5 ± 0.1 (21 ± 1%) |
| 18 | 3-CH2CH2COOH | 5-CH3 | Naphth-2-yl | 0.80 ± 0.02 (25 ± 1%) | 0.21 ± 0.01 (14 ± 1%) | 3.3 ± 0.1 (23 ± 1%) | i.a. |
| 19 | 3-CH2CH2COOH | 6-CH3 | Naphth-2-yl | 7.4 ± 0.4 (15 ± 1%) | 6.3 ± 0.9 (20 ± 1%) | 11 ± 2 (2.5 ± 0.2%) | i.a. |
| 20 | 3-CH2CH2COOH | 4-Cl | Naphth-2-yl | 3.0 ± 0.1 (16 ± 1%) | 1.9 ± 0.2 (16 ± 1%) | 1.4 ± 0.1 (6.0 ± 0.1%) | i.a. |
| 21 | 3-CH2CH2COOH | 4-OH | Naphth-2-yl | 5.3 ± 0.5 (54 ± 3%) | 2.4 ± 0.1 (34 ± 1%) | 2.3 ± 0.3 (20 ± 2%) | 4.5 ± 0.1 (16 ± 1%) |
| 22 | 3-CH2CH2COOH | 4-OCH3 | Naphth-2-yl | 0.30 ± 0.04 (43 ± 2%) | 0.06 ± 0.01 (32 ± 1%) | 1.3 ± 0.1 (27 ± 1%) | 11 ± 3 (22 ± 1%) |
| 23 | 3-CH2CH2COOH | 4-OCF3 | Naphth-2-yl | 1.1 ± 0.3 (31 ± 4%) | 0.38 ± 0.06 (30 ± 2%) | 1.0 ± 0.2 (15 ± 1%) | 1.1 ± 0.1 (12 ± 1%) |
| 24 | 3-CH2CH2COOH | 4-tBu | Naphth-2-yl | 0.31 ± 0.08 (63 ± 8%) | 0.27 ± 0.02 (25 ± 1%) | 1.5 ± 0.1 (20 ± 1%) | 0.30 ± 0.01 (13 ± 1%) |
| 25 | 3-CH2CH2COOH | 5-Cl | Naphth-2-yl | 0.9 ± 0.2 (42 ± 2%) | 0.28 ± 0.03 (17 ± 1%) | 6 ± 1 (26 ± 2%) | 2.6 ± 0.4 (12 ± 1%) |
| 26 | 3-CH2CH2COOH | 5-OH | Naphth-2-yl | 8.9 ± 0.8 (28 ± 2%) | 10 ± 2 (24 ± 2%) | 13 ± 1 (9 ± 1%) | i.a. |
| 27 | 3-CH2CH2COOH | 5-OCH3 | Naphth-2-yl | 0.23 ± 0.04 (53 ± 2%) | 0.36 ± 0.01 (23 ± 1%) | 2.0 ± 0.1 (15 ± 1%) | i.a. |
| 28 | 3-CH2CH2COOH | 5-OCF3 | Naphth-2-yl | 0.21 ± 0.02 (54 ± 1%) | 0.10 ± 0.01 (19 ± 1%) | 1.1 ± 0.2 (18 ± 2%) | i.a. |
| 29 | 3-CH2CH2COOH | 4,5-Methylenedioxy | Naphth-2-yl | 0.46 ± 0.03 (25 ± 1%) | 0.4 ± 0.1 (31 ± 3%) | 0.9 ± 0.3 (15 ± 2%) | 2.9 ± 0.6 (14 ± 1%) |
| 30 | 3-CH2CH2COOH | — | 1,1'-Biphenyl-4-yl | >30 | 2.5 ± 0.5 (38 ± 4%) | 1.6 ± 0.2 (16 ± 1%) | 1.3 ± 0.2 (33 ± 1%) |
| 31 | 3-CH2CH2COOH | — | 4-(Furan-2-yl)phenyl | 1.8 ± 0.3 (22 ± 1%) | 0.20 ± 0.01 (43 ± 1%) | 2.2 ± 0.1 (9 ± 1%) | 5.4 ± 0.1 (23 ± 1%) |
| 32 | 3-CH2CH2COOH | — | 4-(Furan-3-yl)phenyl | 1.6 ± 0.3 (31 ± 2%) | 2.4 ± 0.2 (57 ± 3%) | 4.2 ± 0.9 (38 ± 5%) | 0.23 ± 0.01 (20 ± 1%) |
| 33 | 3-CH2CH2COOH | — | 4-(Thiophen-2-yl)phenyl | 1.5 ± 0.1 (27 ± 1%) | 0.64 ± 0.06 (35 ± 1%) | 1.4 ± 0.2 (11 ± 1%) | 1.7 ± 0.4 (21 ± 1%) |
| 34 | 3-CH2CH2COOH | — | 4-(Thiophen-3-yl)phenyl | 1.7 ± 0.1 (32 ± 1%) | 1.6 ± 0.3 (59 ± 5%) | 1.3 ± 0.2 (17 ± 1%) | 0.6 ± 0.1 (18 ± 1%) |
| 35 | 3-CH2CH2COOH | — | 4-(Isoxazol-3-yl)phenyl | 8.0 ± 0.4 (11 ± 1%) | 5.6 ± 0.4 (29 ± 1%) | 5.8 ± 0.8 (30 ± 2%) | 6.8 ± 0.6 (32 ± 1%) |
| 36 | 3-CH2CH2COOH | — | 4-(Isoxazol-4-yl)phenyl | Unstable | Unstable | Unstable | Unstable |
| 37 | 3-CH2CH2COOH | — | 4-(Oxazol-4-yl)phenyl | 20 ± 1 (36 ± 3%) | 12 ± 1 (52 ± 2%) | 6 ± 1 (15 ± 1%) | 6.9 ± 0.3 (41 ± 1%) |
| 38 | 3-CH2CH2COOH | — | 4-(Oxazol-5-yl)phenyl | > 30 | 12 ± 1 (32 ± 3%) | 5.3 ± 0.2 (16 ± 1%) | 15 ± 4 (53 ± 4%) |
| 39 | 3-CH2CH2COOH | — | 4-(1,2,3-Triazol-1-yl)phenyl | i.a. | i.a. | >30 | i.a. |
| 40 | 3-NHCH2COOH | 4-OCH3 | 4-(Furan-3-yl)phenyl | >30 | 1.6 ± 0.1 (54 ± 2%) | 7.7 ± 0.5 (9 ± 1%) | 0.29 ± 0.01 (43 ± 1%) |
| 41 | 3-CH2CH2COOH | 4-tBu | 4-(Furan-2-yl)phenyl | 0.17 ± 0.04 (72 ± 5%) | 0.14 ± 0.01 (34 ± 1%) | 2.4 ± 0.1 (16 ± 1%) | 0.015 ± 0.002 (19 ± 1%) |
Results are expressed as the mean ± SEM; n = 3. Maximum relative activation (max. rel. act.) refers to the activity of reference compounds GW7647 (PPARα), rosiglitazone (PPARγ), L165,041 (PPARδ) at 1 µM, and GW4064 (FXR) at 3 µM.
i.a. inactive at 10 μM, tox. toxic at 30 μM.
