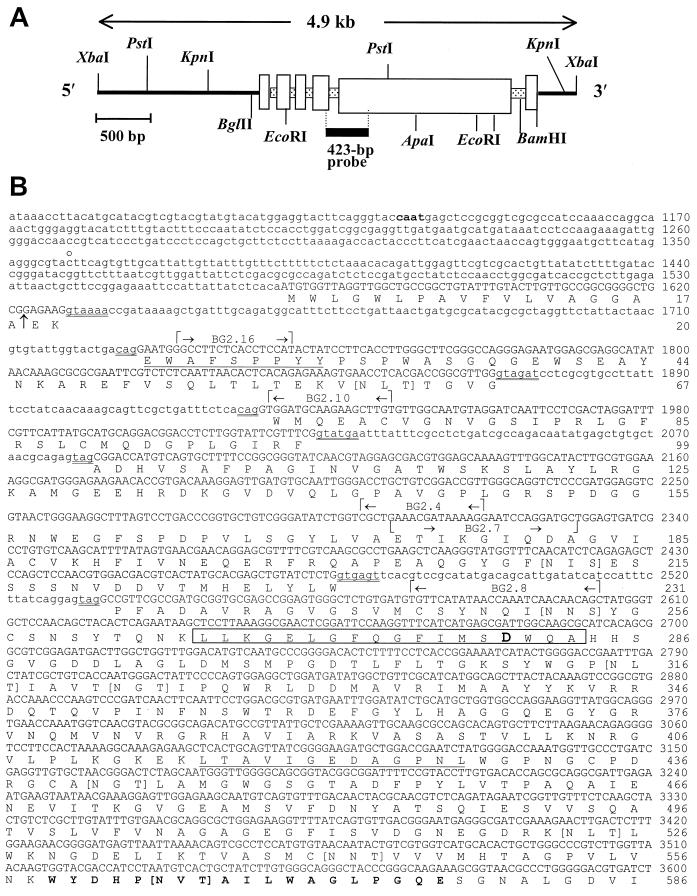
FIG. 2.
Restriction map of 4.9-kb λ phage insert digested with XbaI and subcloned into pZErO (A), and nucleotide sequence of C. immitis BGL2 gene and deduced amino acid sequence (B). The 423-bp probe in panel A is derived from PCR product (a) in Fig. 1. The two underlined amino acid sequences represent matched peptide sequence of the Lys-C-digested, native 120-kDa glycoprotein. The amino acid sequence in boldface type (aa 559 to 577) matched the peptide sequence of Lys-C-digested recombinant BGL2. The boxed sequence represents the 18-aa signature motif of family 3 glycosyl hydrolases. The aspartic acid residue within this sequence (boldface type) is the putative active site. Residues contained within square brackets are putative N glycosylation sites. The arrow between aa 18 and 19 indicates a putative cleavage site of the signal peptide. The double-underlined nucleotide sequences indicate conserved 5′-3′ sequences of introns. The gene-specific primers used for RACE and RT-PCR are indicated. The putative CAAT box (boldface type), 5′ end of the UTR (c̊), stop codon (asterisk), and putative poly(A) addition sites (•) are also indicated.