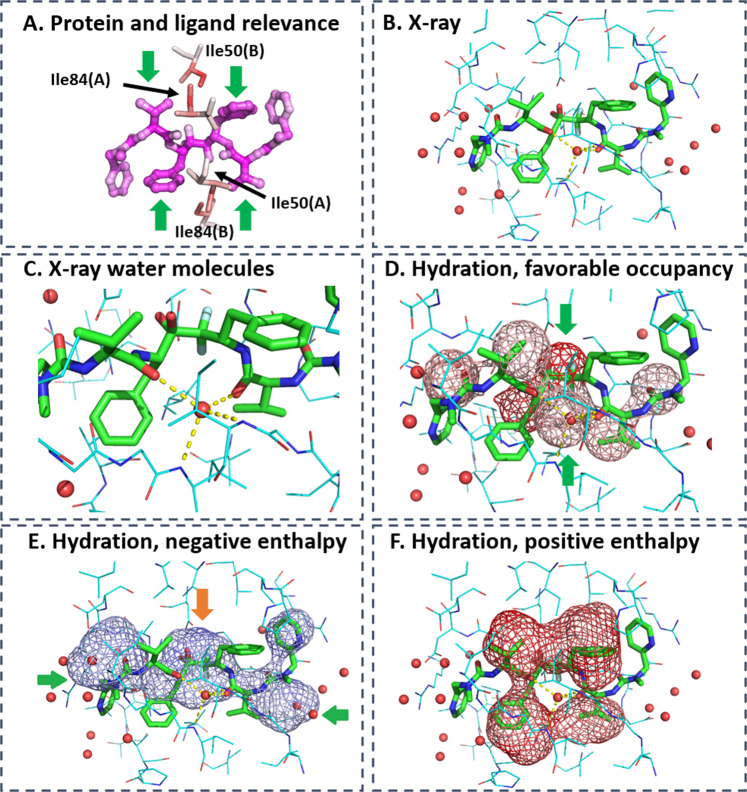
Fig. 6. LRP analysis on HIV-1 protease in complex with inhibitor A79285.
a Higher relevance is highlighted by darker red color for binding site residues (shown as sticks) and by darker magenta color for ligand atoms. b X-ray structure of complex of protein (lines, carbon in blue) and ligand (sticks, carbon in green) with surrounding water molecules (red spheres). PDB-code: 1dif63. c Water-mediated interaction between protein and ligand. d LRP analysis on water occupancy showing relevant water density, in particular around the center of the ligand (green arrows). The lower hydration density overlaps with the water molecule engaged in mediating interactions between ligand and protein. The upper hydration density coincides with a water molecule that is replaced by two hydroxyl groups forming critical and very stable interactions with aspartate side chains of the protein. e This particular water density was predicted with negative, that is, favorable enthalpy (orange arrow), in the LRP analysis focusing on negative enthalpy. First-shell water molecules with favorable enthalpy are additional relevant hydration features (green arrows). f Relevant features with positive enthalpy overlap with hydrophobic ligand moieties (isopropyl and phenyl groups) highlighting the importance of desolvation to ligand binding. Darker color means higher relevance in predicting native binding pose.