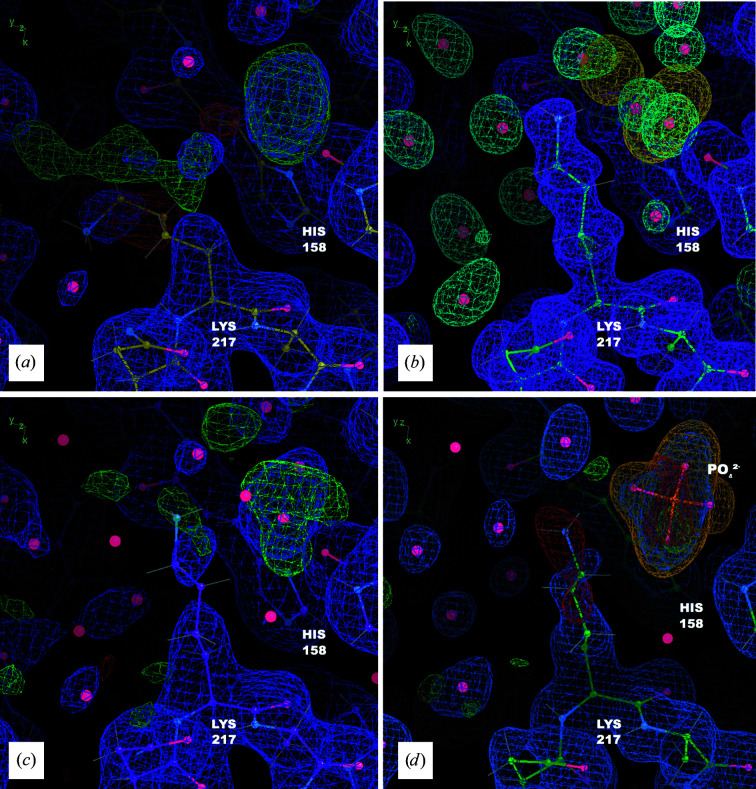
Figure 3.
The MD–MX procedure guides remodeling of Lys217 and reveals a bound inorganic phosphate. (a) Coordinates, 2F o − F c density (blue, 1σ isosurface) and F o − F c density (positive in green and negative in red, 3σ isosurface) from S. (b) Coordinates from M all, with MD protein (purple, 1σ isosurface), solvent (blue, 3σ isosurface) and chloride density (yellow, 10σ isosurface), from the 90–100 ns segment of the simulation; the MD simulation suggests a different conformation for the side chain and a number of ordered waters; it also includes a spot of chloride density in the same position as the positive difference density in (a). (c) Coordinates, 2F o − F c density (blue, 1σ isosurface) and F o − F c density (positive in green and negative in red, 3σ isosurface) from R i; the shape of the difference density is suggestive of a coordinated free phosphate molecule. (d) Coordinates, 2F o − F c density (blue, 1σ isosurface) and F o − F c density (positive in green and negative in red, 3σ isosurface) from model R f refined against the high-resolution data (PDB entry 7v0g): the revised side-chain conformation, water network and phosphate are plausible and improve the difference density in the region. His158 is also shown as a reference point. Polder OMIT map density for the phosphate is shown at a level of 5σ (orange) confirming that the addition of this molecule is reasonable.