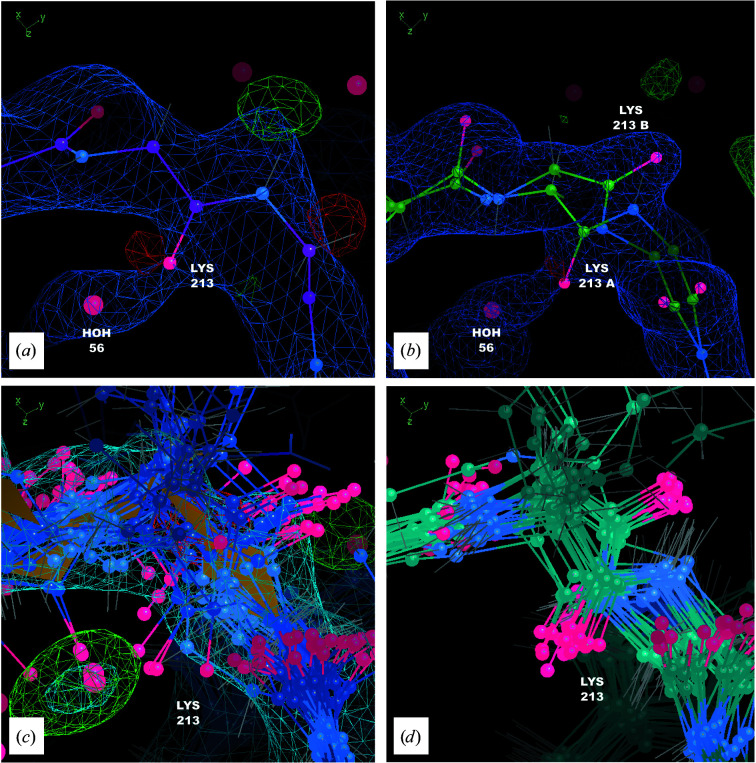
Figure 7.
The MD–MX procedure yields a multi-conformer model of Lys213. (a) Initial MD-revised model (R i) coordinates (magenta), 2F o − F c density (blue, 1σ isosurface) and F o − F c density (positive in green, negative in red, 3σ isosurface). (b) Final MD-revised model (R f) coordinates (green), 2F o − F c density (blue, 1σ isosurface) and F o − F c density (positive in green, negative in red, 3σ isosurface) from refinement against the high-resolution data: the A conformation is at 46% occupancy, the B conformation at 54% occupancy and water 56 in chain S at 100% occupancy. (c) Coordinates from ensemble refinement model (E; blue), 2F o − F c density (blue, 1σ isosurface) and F o − F c density (positive in green, negative in red, 3σ isosurface). (d) Coordinates from the ensemble snapshot from MD simulation (turquoise): the ensemble snapshot suggests a clear multi-conformer state defined by a peptide flip.