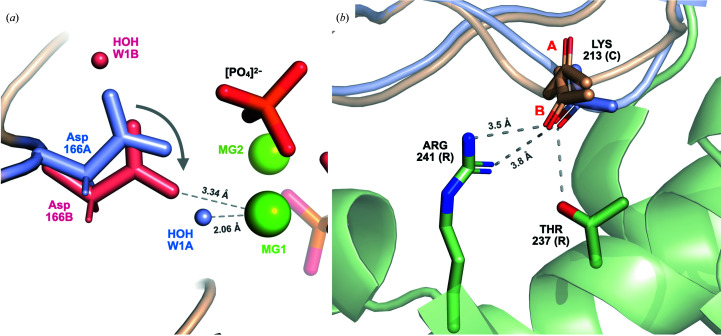
Figure 8.
Implications of the MD-revised structure for PKA-C mechanisms. (a) The alternative conformation of Asp166 suggests a mechanism for the progression of catalysis. In the A conformation (blue), Asp166A is shifted away from MG1 and water O atom HOH W1A is coordinated to MG1. In the B conformation (red), Asp166B is shifted down towards MG1 and makes room for water O atom HOH W1 to occupy a different site above the side chain (where it would have clashed with conformation A of Asp166). The distance between OD2 of Asp166B and MG1 is larger (3.34 Å) than the distance between the O atom of water HOH 1A in chain W and MG1 (2.06 Å), potentially weakening coordination to MG1 and encouraging its escape from the active site post-catalysis. The multiple conformations suggest the possibility of a concerted motion (arrow) for Asp166 associated with progression of the phosphotransfer reaction. (b) The alternative conformation of Lys213 is consistent with the backbone pose for binding to the regulatory subunit. The final MD-revised structure (R f, light brown) models Lys213 of the catalytic subunit (PKA-C) with two conformers defined by a peptide flip (side chain not shown). The B conformer of Lys213 is consistent with the backbone O-atom orientation of PKA-C bound to the regulatory subunit RIα (PDB entry 2qcs, with the catalytic subunit shown in light blue and the regulatory subunit shown in light green). The backbone O atom of Lys213(C) is close to Thr237 and Arg241(R).