Figure 2.
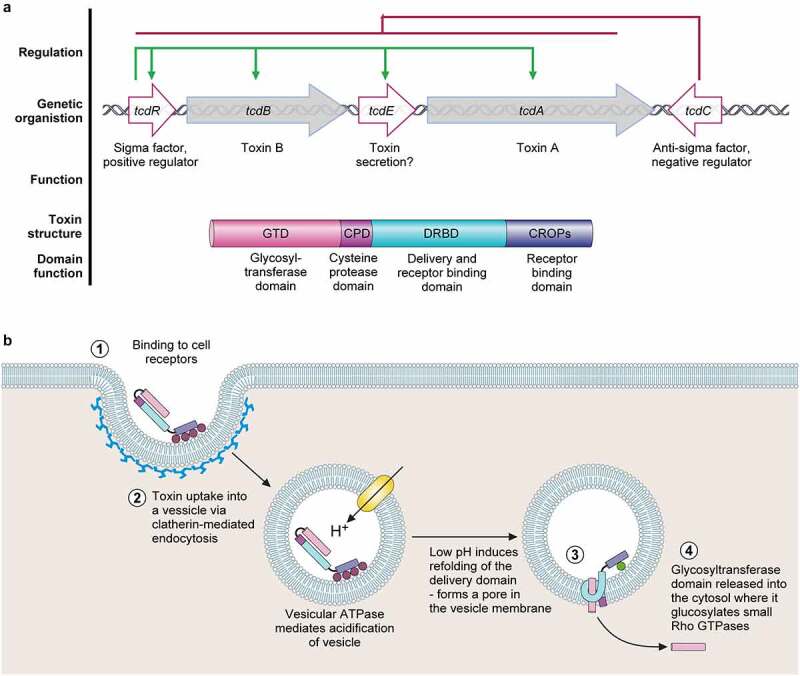
The pathogenicity locus and toxin mode of action. .
a. The pathogenicity locus (PaLoc) is composed of 5 genes: tcdA and tcdB, encoding toxins A and B respectively; tcdR, encoding an alternative sigma factor and likely positive regulator of the PaLoc (regulation shown above in green); tcdE, encoding a holin-like protein putatively involved in toxin secretion; and tcdC, an anti-sigma factor and negative regulator of the PaLoc genes (regulation shown above in red). Toxins A and B both consist of a broadly similar four-domain structure. At the N-terminal, the glucosyltransferase domain (GTD) is the active toxin moiety which inactivates members of the Rho GTPase family. A cysteine protease domain is next to the GTD, and is involved in auto-processing and release of the GTD. The next domain, often called the Delivery and Receptor Binding Domain (DRBD), contains a hydrophobic region and is thought to be involved in translocation of the GTD from the lumen of endocytic vesicles into the host cell cytoplasm. The final C-terminal receptor-binding domain (also known as C-terminal combined repetitive oligopeptides (CROPS) domain) binds to a range of cellular receptors.b. Toxin mode of action [65]. The toxins bind to various cellular receptors via the C-terminal CROPs domain, triggering clathrin-dependent endocytosis (1) followed by acidification of the resulting vesicle (2). The drop in pH triggers a conformational change in the delivery domain which inserts into, and forms a pore in, the vesicle membrane, through which the GTD transits into the host cytoplasm (3). The GTD is then released by a cleavage event mediated by the cysteine protease domain, in a process that is dependent on host inositol hexakisphosphate (4). The GTD is then able to glucosylate and inactive members of the small Rho GTPase family, including Rho, Rac, and Cdc42. Inactivation of Rho GTPases results in multi-level cellular disruption, including dysregulation of actin depolymerization, which causes disruption of tight junctions and loss of intestinal barrier function, induction of proinflammatory cytokines and activation of programmed cell death.
