Figure 4. mAb 770F7 defines a novel epitope on EBV gH D-IV.
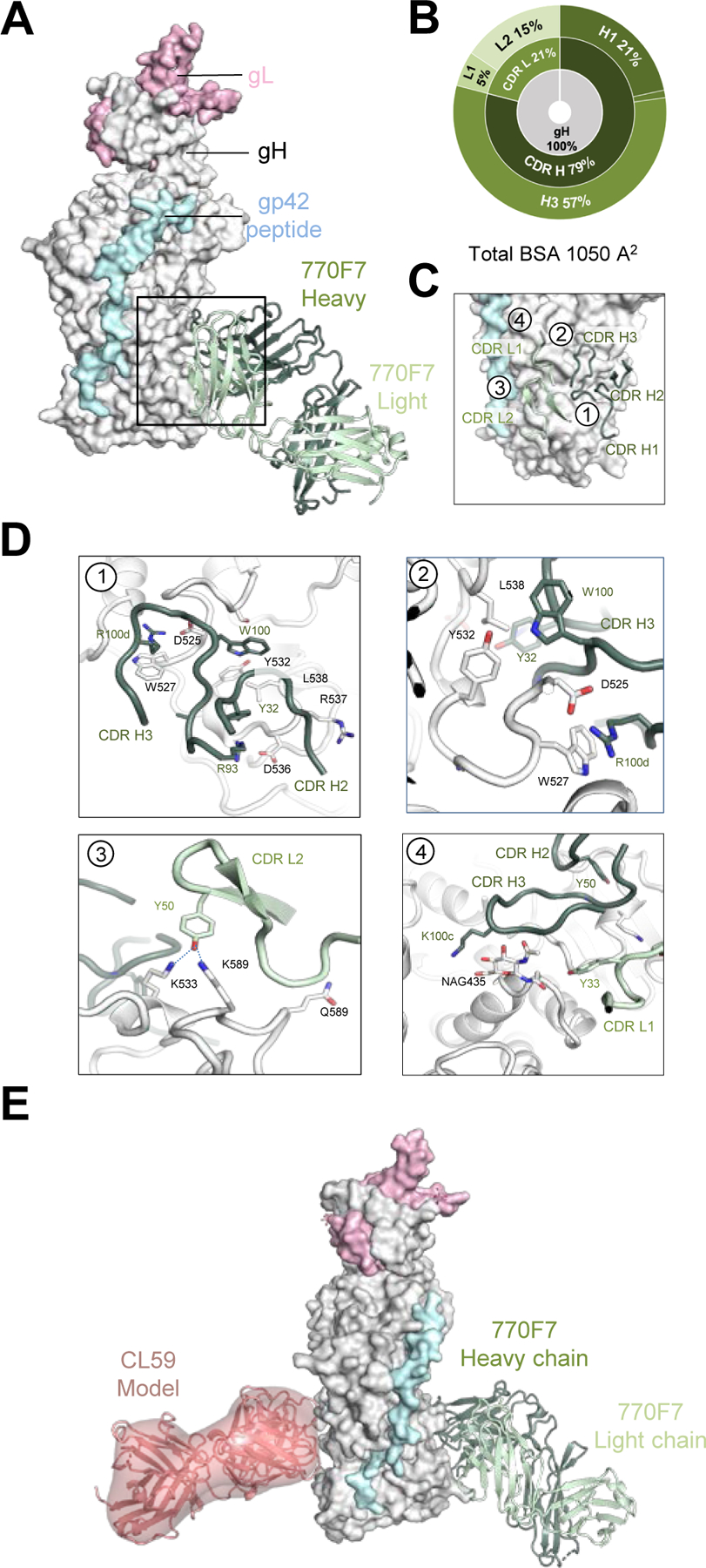
(A) Structure of 770F7 Fab in complex with EBV gH/gL/gp42peptide (surface representation; gH, gL, and gp42p are colored in light grey, light pink, and pale cyan respectively) are shown bound to mAb 770F7 (ribbon representation, heavy chain and light chain are colored with forest green and pale green respectively).
(B) CDR-glycoprotein epitope BSA % are shown represented in pie-chart format. The 770F7 epitope is on the gH molecule (inner circle), with both heavy and light chain recognition of gH (middle), with predominant recognition by the CDR H2 and H3 loops (outer).
(C) Major antibody loops involved in gH/gL recognition are shown in ribbon representation with gH/gL in surface representation.
(D) mAb 770F7 contact residues are shown in stick representations based on (1) CDR H2, and H3; (2) CDR H3; (3) CDR L2; (4) CDR H3 and CDR L1.
(E) Structure model of 770F7 and CL59 in complex with gH/gL/gp42p (surface representation); 770F7 Fab is shown in ribbon representation colored as in A, and CL59 Fab is shown as both cartoon and surface representation in salmon color.
