Abstract
Quercetin, a flavonol present in many vegetables and fruits, has been identified as a chemoprevention agent in several cancer models. However, the molecular mechanism of quercetin’s anticancer activity is not entirely understood. MicroRNAs (miRNAs), small noncoding RNAs, have been reported to play key roles in various biological processes by regulating their target genes. We hypothesized that quercetin can exert an anticancer effect through the regulation of miRNAs. To test this hypothesis, we investigated the effects of quercetin on the expression of tumor-suppressive miRNAs in cervical cancer. Quercetin up-regulated the in vivo and in vitro expression of tumor-suppressive miRNAs miR-26b, miR-126, and miR-320a. Quercetin suppressed the level of β-catenin, encoded by catenin beta 1 (CTNNB1), by up-regulating miR-320a in HeLa cells. Moreover, quercetin increased the expression of mir-26b, mir-126, and mir-320a precursors in HeLa cells. The results from this study show that quercetin has the potential to prevent cervical cancer by regulating the expression of tumor-suppressive miRNAs.
Keywords: quercetin, cervical cancer, microRNA, β-catenin
INTRODUCTION
Quercetin (3,3′,4′,5,7′-pentahydroxyflavone), one of the plant flavonols in vegetables and fruits, exhibits antiatherogenic [1], anti-obesity [2], and neuroprotective [3] effects. Compared to other flavonols, quercetin has been reported to have anticancer activity in various cancer cells [4,5,6,7]. Recent research has demonstrated that quercetin exerts cytotoxic effects via different molecular mechanisms, including the inhibition of cell growth and induction of cell death [8,9,10]. These studies have revealed the molecular mechanism of quercetin’s anticancer activity.
Several studies have shown that dietary polyphenols can regulate the expression of microRNAs (miRNAs) [11,12,13], which are short, noncoding, single-stranded RNAs of approximately 22 nucleotides (nts). MiRNAs are involved in many biological processes, such as the cell cycle [14], cell death [15], and several developmental and physiological processes. Some miRNAs are differentially expressed in cancer cells; their abnormal expressions lead to cancer development [16,17,18]. It has been reported that miR-26b, miR-126, miR-320, and miR-744 suppress human cervical cancer cell proliferation, migration, and invasion [19,20,21,22]. Therefore, the regulation of miRNA expression could be an effective strategy for cancer therapy.
The primary miRNA (pri-miRNA) transcripts are cleaved into approximately 70-nt stem-loop precursor miRNAs (pre-miRNAs) by the nuclear RNase III Drosha and further processed to mature miRNAs by cytosolic Dicer, another RNase-III-related enzyme [23]. Although miRNAs have been discovered to have diverse roles, their most typical and important function involves the posttranscriptional regulation of target gene mRNA by binding to the 3′-untranslated region (3′-UTR) of the mRNA transcript. The mRNAs bound to miRNAs become either less efficient in translation or degraded [24]. As an miRNA does not necessarily bind to its target mRNA completely, a single miRNA can target multiple mRNAs.
Our previous study reported that epigallocatechin-3-O-gallate (EGCG), a major polyphenolic compound in green tea, increased Let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells [25]. Because the health benefits of quercetin are diverse and functions of miRNAs result in diverse biological consequences, we hypothesized that anticancer effect of quercetin may involve the modulation of miRNA expression. Cervical cancer is one of the most common malignances among women globally. Although the antiproliferative and pro-apoptotic potentials of quercetin in HeLa cells is known [26], the effect of quercetin on miRNA expression in human cervical cancer remains unknown. The purpose of this study was to clarify the relationship between the typical flavonol quercetin and miRNAs in human cervical cancer cells and to demonstrate new potentials of the mechanism of action of flavonoids.
MATERIALS AND METHODS
Cell lines and reagents
HeLa cells (American Type Culture Collection; ATCC) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS; Gibco) in a state of logarithmic growth at 37°C in a humidified chamber with 5% CO2. The effects of quercetin were examined by harvesting cells culture plates and treating them with quercetin at the indicated concentrations in DMEM supplemented with 2% FBS for the period. Quercetin (Nacalai Tesque) was dissolved in dimethyl sulfoxide (DMSO) and stored at −30°C until use. The target synthetic microRNA inhibitor of hsa-miR-320a-5p (catalog no. 4464084) and negative control inhibitor (catalog no. 4464076) were purchased from Ambion. Monoclonal anti-β-catenin antibody (catalog no. 610154) was purchased from BD Biosciences, and monoclonal anti-β-actin antibody (catalog no. A5441) was purchased from Sigma-Aldrich.
Animals and tumor model of cervical cancer
In Experiment 1, 6-week-old female BALB/c nude mice (Kyudo Company, Saga, Japan) were inoculated subcutaneously in the intrascapular area with 1.2 × 107 HeLa cells. After tumor formation, the mice were divided randomly into three groups with an even distribution of tumor sizes. Mice were orally administered 200 µL of vehicle (dH2O containing 1.5% DMSO) or quercetin (10 mg/kg body weight [b.w.] or 50 mg/kg b.w.) at 48 hr intervals. Eleven days after administration, the RNA of the cervical tumors was extracted.
In Experiment 2, 6-week-old female C57BL6/J mice (Kyudo Company, Saga, Japan) were randomly assigned to a control group to receive oral administration of the vehicle alone or a quercetin group to receive quercetin at 50 mg/kg b.w. in a 200 µL volume. The RNA of the plasma was extracted 48 hr after administration.
Experiments 1 (approval number A30-041-5) and 2 (approval number A30-042-4) were carried out according to the guidelines for animal experiments at the Faculty of Agriculture, Kyushu University. All animals were given free access to the American Institute of Nutrition 93 growth (AIN-93G) diet (Oriental Yeast Co., Ltd). All animal experiments were approved by the Animal Care and Use Committee of Kyushu University, Fukuoka, Japan.
Real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissues or cell samples using TRIzol (Invitrogen). Complementary DNA (cDNA) was synthesized from the total RNA using miRCURY LNATM RT kit (Qiagen). qRT-PCR was performed using the miRCURY LNATM SYBR Green PCR kit (Qiagen) and a CFX96 real-time PCR analysis system (Bio-Rad). LNATM PCR primer mix, hsa-miR-26b-5p (GeneGlobe ID: YP00204172), hsa-miR-126-3p (GeneGlobe ID: YP00204227), and hsa-miR-320a (GeneGlobe ID: YP00206042), and hsa-mir-744 (GeneGlobe ID: YP00204663) were purchased from Qiagen. The miRNA expression was normalized to that of the U6 small nuclear RNA (Qiagen, #YP00203907). Plasma miRNA expression was normalized to the spike-in control (Qiagen).
For precursor miRNA analysis, cDNA was synthesized from total RNA with a miScript II RT Kit (Qiagen). The expression levels of precursor miRNA were determined using a miScript SYBR Green PCR Kit (Qiagen). cDNA was used as a template for quantitative real-time PCR with an Hs-mir-26b-1-PR miScript Precursor Assay (#MP00001694), Hs-mir-126-1-PR miScript Precursor Assay (#MP00000476), and Hs-mir-320a-1-PR-miScript Precursor Assay (#MP00001869; Qiagen). Pre-miRNA expression was normalized to the control RNA (SNORD68, Qiagen; GeneGlobe ID: MS00033712).
Western blot analysis
Cells were lysed in 1% Triton X-100 lysis buffer consisting of 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM ethylenediamine tetraacetic acid (EDTA), 50 mM NaF, 30 mM Na4P2O7, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 µg/mL aprotinin, and 1% Triton X-100. Approximately 50 µg of protein was suspended in Laemmli sample buffer consisting of 0.1 M Tris-HCl buffer (pH 6.8), 1% sodium dodecyl sulfate (SDS), 0.05% 2-mercaptoethanol, 10% glycerol, and 0.001% bromophenol blue, boiled, and electrophoresed on 8% SDS-polyacrylamide gels. The gels were then transferred onto Trans-Blot nitrocellulose membranes (Bio-Rad). After blocking the blots in Tween 20/Tris Buffered Saline (TTBS) containing 2.5% bovine serum albumin, the proteins were identified using the indicated antibodies in the same solution. Membranes were washed with TTBS and incubated with anti-rabbit or anti-mouse horseradish peroxidase (HRP) conjugates. The specific bands were detected using an enhanced chemiluminescence system according to the manufacturer’s instructions (Amersham Biosciences).
miRNA inhibitor transfection
The miR-320a inhibitor and negative control inhibitor were transfected into cells with LipofectamineTM RNAiMAX (Invitrogen) according to the manufacturer’s instructions. DMEM, RNA reagents, and RNAiMAX were mixed with a pipette, and the mixture was allowed to sit for 10 min at room temperature before finally being added to the cells. After transfection for 48 hr, the cells were treated with 5 μM quercetin in DMEM supplemented with 2% fetal bovine serum (FBS) for 72 hr.
Statistical analyses
The data are presented as means ± standard error of the mean (SEM). The data were analyzed with GraphPad Prism (version 4). Student’s t-test was used for comparing two conditions, and Dunnett’s test was used for comparing with the control. A p value of less than 0.05 was considered statistically significant.
RESULTS
Dietary quercetin up-regulates tumor-suppressive miRNA expression in the uterine cervical tumor
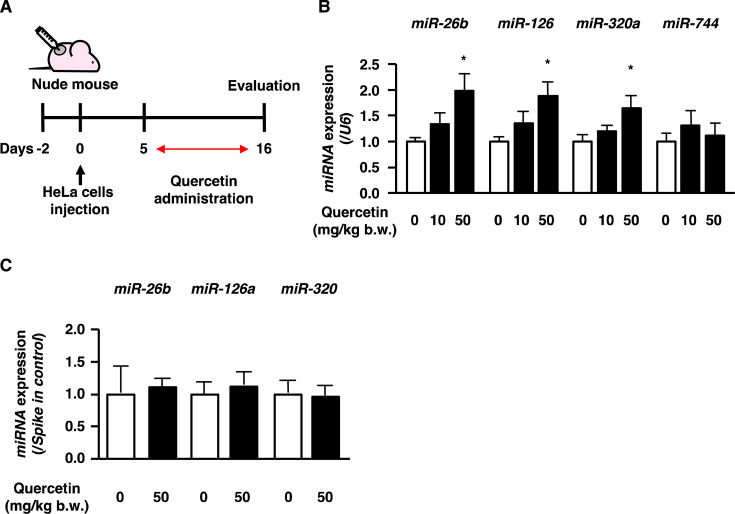
Quercetin has been known as an effective compound for treating cervical cancer [4]. MiR-26b, miR-126, miR-320, and miR-744 have been reported to suppress the growth, metastasis, and infiltration of human cervical cancer cells [19,20,21,22]. The effect of quercetin on the expression of miR-26b, miR-126, miR-320a, and miR-744 in human cervical cancer tumors was examined in 6-week-old BALB/c nude mice subcutaneously transplanted with HeLa cells (Fig. 1A). After the tumors became palpable, the mice were orally administered quercetin at 10 mg/kg or 50 mg/kg b.w. once every two days. After 11 days of treatment, all the mice were sacrificed. At the time of tumor sample collection, tumor growth was slower than expected, and regression was observed in a few controls; therefore, the antitumor effect of quercetin could not be determined. However, the expression of miR-26b, miR-126, and miR-320a (human miR-320) in the early stage of cervical cancer was up-regulated in the 50 mg/kg b.w. quercetin treatment groups (Fig. 1B). Measurement of miR-744 expression in the tumors revealed that quercetin administration did not affect the expression of miR-744 (Fig. 1B).
Fig. 1.
Dietary quercetin up-regulates tumor-suppressive microRNA (miRNA) expression in uterine cervical tumors.
(A, B) Nude mice implanted with HeLa cells. After the formation of tumors, mice were administered quercetin orally (10 or 50 mg/kg b.w./2 days). Eleven days after administration, miRNA expression was measured in the uterine cervix tumors. Results represent means ± SEM (n = 6–10). Asterisks indicate statistical significance to 0 mg/kg b.w. quercetin treatment calculated. *p<0.05. (C) C57BL/6J mice were administered quercetin orally (50 mg/kg b.w.). At 48 hr after administration, the expression of miR-26b, miR-126, and miR-320 was measured in plasma. Results represent means ± SEM (n=6).
MiRNAs can be released into the extracellular space, work locally, or enter the circulation to act at distal sites. Thus, we hypothesized that quercetin induced miRNA secretion from tissues other than tumors and that secreted miRNA could be taken up into tumors. We examined whether quercetin induced miRNA secretion by testing quercetin’s effect on the plasma expression of the miRNAs in normal mice. A single-dose administration of quercetin did not affect miR-26b, miR-126a (also called mouse miR-126), or miR-320 expression in the plasma (Fig. 1C). These results suggest that quercetin increases the expression of tumor-suppressive miRNA molecules in cervical cancer tumors.
Quercetin up-regulates tumor-suppressive miRNAs expression in cervical cancer cells
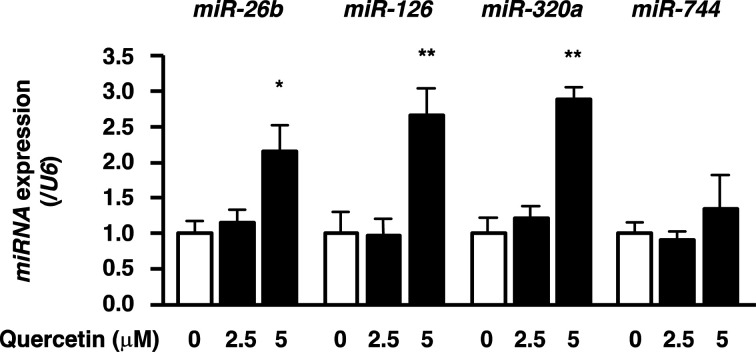
The effect of quercetin on miRNA expression in vitro was determined by treating HeLa cells with quercetin. qRT-PCR analysis confirmed that quercetin increased miR-26b, miR-126, and miR-320a expression in cervical cancer cells without influencing miR-744 expression, just like in cervical tumors (Fig. 2). These results indicate that quercetin directly interacts with the cells and promotes miRNA up-regulation.
Fig. 2.
Quercetin up-regulates tumor-suppressive microRNA (miRNA) expression in cervical cancer cells.
HeLa cells were treated with the indicated concentrations of quercetin for 24 hr, and then miRNA expression was measured by qRT-PCR. Results represent means ± SEM from three independent experiments (n=3). Asterisks indicate statistical significance to 0 μM quercetin treatment calculated. *p<0.05; **p<0.01.
Quercetin suppresses the β-catenin level by modulating miR-320a activities
MiR-320a has several target genes related to tumor progression, including the gene encoding β-catenin. β-catenin, encoded by CTNNB1, functions as an oncoprotein when it is translocated to the nucleus. β-catenin and the activation of the Wnt pathway have been reported to play an important role in cancer progression. Online prediction of the target gene by microRNA.org showed that the targeted binding site was between miR-320 and the 3′-UTR of β-catenin, and transfection of a miR-320 mimic significantly lowered β-catenin expression in chondrocytes [27]. Hence, quercetin was found to decrease the level of β-catenin in HeLa cells here (Fig. 3A). We used an anti-miR-320a nucleotide to inhibit endogenous miR-320a activities to assess whether the up-regulation of miR-320a by quercetin was involved in this effect. Quercetin was observed to decrease the β-catenin protein level in the control cells, whereas miR-320a inhibition attenuated the effect of quercetin (Fig. 3B). These results demonstrate that quercetin decreases the β-catenin level by modulating miR-320a expression.
Fig. 3.
Quercetin suppresses the β-catenin level by modulating miR-320a activities.
(A) β-catenin level in HeLa cells treated with quercetin for 72 hr. (B) The β-catenin level was measured when HeLa cells were transfected with 10 nM miR-320a inhibitor for 48 hr and then treated with 5 μM quercetin for 72 hr. Results represent means ± SEM from three independent experiments (n=3). Asterisks indicate statistical significance to 0 μM quercetin treatment calculated. *p<0.05; **p<0.01.
Quercetin promotes tumor-suppressive miRNA precursor expression
More than half of miRNA genes have their own promoters to facilitate the independent expression of miRNA genes. After transcription, cleavage, and processing, the mature miRNA is transported from the nucleus to the cytoplasm. The transcriptional regulation of precursor miRNA molecules is important for controlling miRNA expression. In this study, we hypothesized that quercetin has direct effects on cancer cells that increase miRNA expression and examined the time course effects of quercetin on pre-mir-26b, pre-mir-126, and pre-mir-320a expression in HeLa cells (Fig. 4). Pre-mir-26b was significantly up-regulated after 24 hr of the treatment with quercetin. Pre-mir-126 was significantly increased after 6 hr of treatment with quercetin. Pre-mir-320a was significantly up-regulated at 12 hr of treatment with quercetin and decreased at 24 hr. These results suggest that quercetin has an inductive effect on the transcription of these miRNA genes.
Fig. 4.
Quercetin promotes tumor-suppressive microRNA (miRNA) precursor expression.
HeLa cells were treated with 5 μM quercetin for 0–24 hr, and then precursor miRNA (pre-mir-26b, pre-mir-126, and pre-mir-320a) expression was measured by qRT-PCR. Results represent means ± SEM from four independent experiments (n=4). Asterisks indicate statistical significance to quercetin 0 hr treatment calculated. *p<0.05; **p<0.01.
DISCUSSION
This study aimed to investigate the effect of quercetin on the expression of tumor-suppressive miRNAs in cervical cancer cells. We found that quercetin up-regulated tumor-suppressive miRNAs miR-26b, miR-126, and miR-320a in uterine cervical tumors as well as cells. Quercetin suppressed the β-catenin level through miR-320a. Moreover, quercetin increased the expression of tumor-suppressive miRNA precursors pre-mir-26b, pre-mir-126, and pre-mir-320a. Therefore, we showed that the up-regulation of tumor-suppressive miRNAs by quercetin may be partially relevant to quercetin’s anticancer effect.
Quercetin inhibits tumor progression in various cancer cells [28]. However, we did not observe the preventive effect of quercetin in cervical tumors. Such discrepancy may be due to a difference in the dose, duration, or administration method of quercetin. On the other hand, quercetin suppressed the β-catenin level via miR-320a in HeLa cells. The transcription factor clusters of β-catenin/Snail1/Twist have been implicated in the process of epithelial-mesenchymal transition [29]. Wnt/β-catenin signaling plays a critical role in the initiation and maintenance of cancer stem cells [30]. Here, the in vitro results suggest that quercetin may inhibit epithelial-mesenchymal transition in cervical cancer progression and cervical cancer stem cell differentiation through a decrease of β-catenin by up-regulating miR-320a.
After absorption, aglycone quercetin is metabolized to the methylated, glucuronidated, or sulfated forms by enterocyte enzymes [31, 32]. As a result, it is difficult to detect the aglycone form of quercetin in the blood. However, considerable amounts still exist within tissues, likely because polyphenol conjugations can be hydrolyzed at the vascular level, producing the aglycone forms in tissues [33]. It is necessary to assess whether quercetin reaches tumor tissue. Hence, it is necessary to confirm whether quercetin reaches tumor tissue in future studies. Feeding quercetin-enriched diets to mice significantly increased plasma quercetin (2.31 μM) [34]. Moreover, pathological conditions, such as cancer and inflammation, may increase the bioavailability of flavonoids [35, 36]. Therefore, quercetin was assumed to increase miRNA expression after intestinal absorption and transport into the target tumor tissue.
The biogenesis of miRNA is tightly regulated, resulting in characteristic miRNA expression patterns for different developmental stages, tissues, and cell types. The expression of miRNAs has been demonstrated to be altered in cancer [16,17,18]. Nevertheless, the signaling pathways that regulate the expression of miRNAs are largely unknown. The transcription of miRNA is known to be regulated by transcriptional factors [37, 38] or epigenetic factors [39,40,41]. Here, quercetin was found to increase pre-mir-126 expression at earlier time points than pre-mir-26b and pre-mir-320a in cervical cancer cells. It is considered that the up-regulation of miRNAs miR-26b, miR-126, and miR-320a by quercetin is under the control of different regulatory mechanisms. Mature miRNA expression can also be regulated by miRNA processing. The flavonoid apigenin has been reported to inhibit the phosphorylation of TAR RNA-binding protein (TRBP) and its subsequent miRNA maturation [42]. It is still unclear whether quercetin regulates miRNA expression at the posttranscriptional level. Further studies are required to clarify the relation between quercetin and miRNA processing.
Recent studies proposed that miRNA may take part in the posttranscriptional regulation of approximately 60% of all human genes and play a fundamental role in modulating gene expression [43]. Here, quercetin was found to decrease the β-catenin level by regulating miR-320a expression. In addition to the β-catenin gene, miR-320a has multiple target genes related to cancer progression, such as neuropilin 1 [44] and Rac1 [45]. Therefore, the quercetin-promoted elevation of miRNA levels may affect many target genes, thus suppressing uterine cervical tumor progression. In this study, we focused on β-catenin, which is the target of miR-320a. It was clarified that the anticancer effect of quercetin is partially involved in the suppression of β-catenin expression via miR-320a. MiR-26b and miR-126 have also been reported to be tumor suppressors that target multiple oncogenes. MiR-26b reduces the migration and invasion abilities of cervical cancer cells by inhibiting Jagged1 (JAG1) expression [20] and suppresses tumor cell growth by targeting prostaglandin-endoperoxide synthase-2 (PTGS2) in breast cancer [46]. MiR-126 inhibits both migration and invasion of cervical cancer cells by regulating zinc finger E-box binding homeobox 1 (ZEB1) [47] and exerts antitumor effects in breast cancer cells by directly targeting AKT2 [48]. It is necessary to clarify how quercetin affects these target molecules downstream by increasing the expression of miR-26b and miR-126.
We have demonstrated up-regulation of the expression of tumor-suppressive miRNAs by quercetin; inhibition of the target genes of the miRNAs may be involved in the protective mechanism of quercetin in cervical cancer. Future studies must determine how quercetin up-regulates the expression of precursor miRNAs in cancer cells.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
Acknowledgments
This research was supported in part by Grants-in-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science to H. Tachibana (grant numbers JP15H02448 and JP20H05683). The authors would like to thank Enago (www.enago.jp) for the English language review.
REFERENCES
- 1.Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, Kobayashi M, Kanayama M, Uchida K, Terao J. 2008. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem 283: 9424–9434. [DOI] [PubMed] [Google Scholar]
- 2.Nabavi SF, Russo GL, Daglia M, Nabavi SM. 2015. Role of quercetin as an alternative for obesity treatment: you are what you eat! Food Chem 179: 305–310. [DOI] [PubMed] [Google Scholar]
- 3.Costa LG, Garrick JM, Roquè PJ, Pellacani C. 2016. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev 2016: 2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almatroodi SA, Alsahli MA, Almatroudi A, Verma AK, Aloliqi A, Allemailem KS, Khan AA, Rahmani AH. 2021. Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways. Molecules 26: 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darband SG, Kaviani M, Yousefi B, Sadighparvar S, Pakdel FG, Attari JA, Mohebbi I, Naderi S, Majidinia M. 2018. Quercetin: a functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J Cell Physiol 233: 6544–6560. [DOI] [PubMed] [Google Scholar]
- 6.Vafadar A, Shabaninejad Z, Movahedpour A, Fallahi F, Taghavipour M, Ghasemi Y, Akbari M, Shafiee A, Hajighadimi S, Moradizarmehri S, Razi E, Savardashtaki A, Mirzaei H. 2020. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci 10: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezzati M, Yousefi B, Velaei K, Safa A. 2020. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci 248: 117463. [DOI] [PubMed] [Google Scholar]
- 8.Maurya AK, Vinayak M. 2015. Quercetin regresses Dalton’s lymphoma growth via suppression of PI3K/AKT signaling leading to upregulation of p53 and decrease in energy metabolism. Nutr Cancer 67: 354–363. [DOI] [PubMed] [Google Scholar]
- 9.Clemente-Soto AF, Salas-Vidal E, Milan-Pacheco C, Sánchez-Carranza JN, Peralta-Zaragoza O, González-Maya L. 2019. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expression‑independent manner in HPV‑positive human cervical cancer‑derived cells. Mol Med Rep 19: 2097–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedhari Sundaram M, Hussain A, Haque S, Raina R, Afroze N. 2019. Quercetin modifies 5’CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J Cell Biochem 120: 18357–18369. [DOI] [PubMed] [Google Scholar]
- 11.Zhou H, Chen JX, Yang CS, Yang MQ, Deng Y, Wang H. 2014. Gene regulation mediated by microRNAs in response to green tea polyphenol EGCG in mouse lung cancer. BMC Genomics 15 Suppl 11: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatadri R, Muni T, Iyer AKV, Yakisich JS, Azad N. 2016. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis 7: e2104–e2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata M, Nonaka H, Komatsu S, Goto M, Morozumi M, Yamada S, Lin IC, Yamashita S, Tachibana H. 2017. Delphinidin prevents muscle atrophy and upregulates MIR-23a expression. J Agric Food Chem 65: 45–50. [DOI] [PubMed] [Google Scholar]
- 14.Rathod SS, Rani SB, Khan M, Muzumdar D, Shiras A. 2014. Tumor suppressive miRNA-34a suppresses cell proliferation and tumor growth of glioma stem cells by targeting Akt and Wnt signaling pathways. FEBS Open Bio 4: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Gu H, Chang J, Wu J, Wang D, Chen S, Yang X, Qian B. 2014. MicroRNA-383 regulates the apoptosis of tumor cells through targeting Gadd45g. PLoS One 9: e110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. 2005. MicroRNA expression profiles classify human cancers. Nature 435: 834–838. [DOI] [PubMed] [Google Scholar]
- 17.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. 2006. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103: 2257–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh A, Lee C, Joseph P, Marchini S, Baccarini A, Kolev V, Romualdi C, Fruscio R, Shah H, Wang F, Mullokandov G, Fishman D, D’Incalci M, Rahaman J, Kalir T, Redline RW, Brown BD, Narla G, DiFeo A. 2014. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial-mesenchymal transition. Nat Commun 5: 2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichikawa R, Kawasaki R, Iwata A, Otani S, Nishio E, Nomura H, Fujii T. 2020. MicroRNA‑126‑3p suppresses HeLa cell proliferation, migration and invasion, and increases apoptosis via the PI3K/PDK1/AKT pathway. Oncol Rep 43: 1300–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Wang W, Wu Y. 2019. MicroRNA-26b acts as an antioncogene and prognostic factor in cervical cancer. Oncol Lett 17: 3418–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi C, Zhang Z. 2017. MicroRNA-320 suppresses cervical cancer cell viability, migration and invasion via directly targeting FOXM1. Oncol Lett 14: 3809–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen XF, Liu Y. 2016. MicroRNA-744 inhibited cervical cancer growth and progression through apoptosis induction by regulating Bcl-2. Biomed Pharmacother 81: 379–387. [DOI] [PubMed] [Google Scholar]
- 23.Kim VN. 2005. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6: 376–385. [DOI] [PubMed] [Google Scholar]
- 24.Ambros V. 2001. microRNAs: tiny regulators with great potential. Cell 107: 823–826. [DOI] [PubMed] [Google Scholar]
- 25.Yamada S, Tsukamoto S, Huang Y, Makio A, Kumazoe M, Yamashita S, Tachibana H. 2016. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci Rep 6: 19225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang T, Fang Y, Wang SX. 2014. Quercetin suppresses HeLa cells by blocking PI3K/Akt pathway. J Huazhong Univ Sci Technolog Med Sci 34: 740–744. [DOI] [PubMed] [Google Scholar]
- 27.Zhang HX, Sun C, Yu HC, Song B, Pan ZX. 2018. Targeted inhibition of β-catenin by miR-320 and decreased MMP-13 expression in suppressing chondrocyte collagen degradation. Eur Rev Med Pharmacol Sci 22: 5828–5835. [DOI] [PubMed] [Google Scholar]
- 28.Tang SM, Deng XT, Zhou J, Li QP, Ge XX, Miao L. 2020. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed Pharmacother 121: 109604. [DOI] [PubMed] [Google Scholar]
- 29.Mahmood MQ, Walters EH, Shukla SD, Weston S, Muller HK, Ward C, Sohal SS. 2017. β-catenin, Twist and Snail: transcriptional regulation of EMT in smokers and COPD, and relation to airflow obstruction. Sci Rep 7: 10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Park SY, Jun Y, Kim JY, Nam JS. 2017. Roles of wnt target genes in the journey of cancer stem cells. Int J Mol Sci 18: 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, da Silva Pinto M. 2013. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol 75: 588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scalbert A, Williamson G. 2000. Dietary intake and bioavailability of polyphenols. J Nutr 130 Suppl: 2073S–2085S. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Vizcaino F, Duarte J, Santos-Buelga C. 2012. The flavonoid paradox: conjugation and deconjugation as key steps for the biological activity of flavonoids. J Sci Food Agric 92: 1822–1825. [DOI] [PubMed] [Google Scholar]
- 34.Huebbe P, Wagner AE, Boesch-Saadatmandi C, Sellmer F, Wolffram S, Rimbach G. 2010. Effect of dietary quercetin on brain quercetin levels and the expression of antioxidant and Alzheimer’s disease relevant genes in mice. Pharmacol Res 61: 242–246. [DOI] [PubMed] [Google Scholar]
- 35.Shimoi K, Saka N, Nozawa R, Sato M, Amano I, Nakayama T, Kinae N. 2001. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab Dispos 29: 1521–1524. [PubMed] [Google Scholar]
- 36.Silberberg M, Gil-Izquierdo A, Combaret L, Remesy C, Scalbert A, Morand C. 2006. Flavanone metabolism in healthy and tumor-bearing rats. Biomed Pharmacother 60: 529–535. [DOI] [PubMed] [Google Scholar]
- 37.Xi Y, Shalgi R, Fodstad O, Pilpel Y, Ju J. 2006. Differentially regulated micro-RNAs and actively translated messenger RNA transcripts by tumor suppressor p53 in colon cancer. Clin Cancer Res 12: 2014–2024. [DOI] [PubMed] [Google Scholar]
- 38.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. 2007. A microRNA component of the p53 tumour suppressor network. Nature 447: 1130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. 2006. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res 66: 1277–1281. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann U, Hasemeier B, Christgen M, Müller M, Römermann D, Länger F, Kreipe H. 2008. Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J Pathol 214: 17–24. [DOI] [PubMed] [Google Scholar]
- 41.Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setién F, Casado S, Suarez-Gauthier A, Sanchez-Cespedes M, Git A, Spiteri I, Das PP, Caldas C, Miska E, Esteller M. 2007. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res 67: 1424–1429. [DOI] [PubMed] [Google Scholar]
- 42.Ohno M, Shibata C, Kishikawa T, Yoshikawa T, Takata A, Kojima K, Akanuma M, Kang YJ, Yoshida H, Otsuka M, Koike K. 2013. The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA103 transgenic mice. Sci Rep 3: 2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman RC, Farh KK, Burge CB, Bartel DP. 2009. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, He X, Liu Y, Ye Y, Zhang H, He P, Zhang Q, Dong L, Liu Y, Dong J. 2012. microRNA-320a inhibits tumor invasion by targeting neuropilin 1 and is associated with liver metastasis in colorectal cancer. Oncol Rep 27: 685–694. [DOI] [PubMed] [Google Scholar]
- 45.Zhao H, Dong T, Zhou H, Wang L, Huang A, Feng B, Quan Y, Jin R, Zhang W, Sun J, Zhang D, Zheng M. 2014. miR-320a suppresses colorectal cancer progression by targeting Rac1. Carcinogenesis 35: 886–895. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Kong X, Zhang J, Luo Q, Li X, Fang L. 2013. MiRNA-26b inhibits proliferation by targeting PTGS2 in breast cancer. Cancer Cell Int 13: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu J, Wang H, Wang H, Chen Q, Zhang L, Song C, Zhou Q, Hong Y. 2019. The inhibition of miR-126 in cell migration and invasion of cervical cancer through regulating ZEB1. Hereditas 156: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sibilano M, Tullio V, Adorno G, Savini I, Gasperi V, Catani MV. 2022. Platelet-derived miR-126-3p directly targets AKT2 and exerts anti-tumor effects in breast cancer cells: further insights in platelet-cancer interplay. Int J Mol Sci 23: 5484. [DOI] [PMC free article] [PubMed] [Google Scholar]