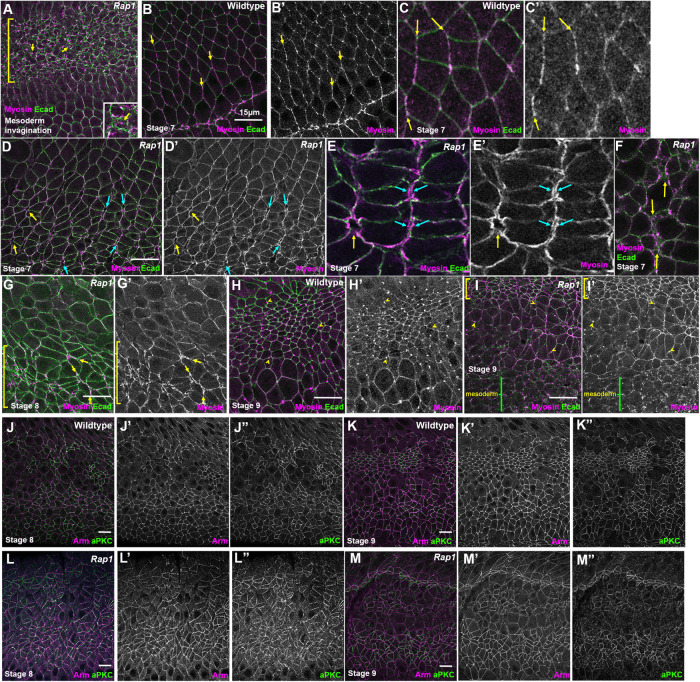
FIGURE 7:
Rap1 RNAi leads to detachment of myosin from many AJs under elevated tension but not to loss of cortical myosin and leads to less-continuous cortical localization of aPKC. (A–I) Embryos expressing Ecad-GFP and mCherry-Myosin. (A) Stage 6. Rap1 RNAi blocks mesoderm invagination. In mesoderm cells (bracket) the myosin network continues to contract but detaches from the AJs (arrows), as we previously observed in Rap1 and cnoM/Z null mutants. (B–F) Stage 7. (B, C) In WT, myosin is enriched at AP borders and is tightly associated with AJs (arrows). (D, E) After Rap1 RNAi, myosin often formed two lines at AP cell junctions (blue arrows) and also appeared to detach from junctions at the center of rosettes (yellow arrows). (F) Tight cortical myosin localization at places where folds were forming was often disrupted. (G) At stage 8, we observed myosin detachment from junctions (yellow arrows) and loss of tight cortical localization in hyperconstricted cells (bracket). (H, I) Stage 9. (H) In WT, myosin is both cortical and strongly enriched at the persistent midbodies (yellow arrows) in dorsal cells that have competed division and is also cortical in more-ventral rounded-up mitotic cells. (I) After Rap1 RNAi, cell shapes are very distorted but myosin remains both cortical and enriched in midbodies. (J–M) aPKC localization. Maximum-Intensity-Projections (MIPs) as aPKC is apical to the AJs. (J, K) WT. Like Arm, aPKC is cortically localized. (L, M) aPKC remains cortical after Rap1 RNAi, but junctional localization appeared less continuous.