Abstract
Puberty is a period of substantial hormonal fluctuations, and pubertal hormones can modulate structural and functional changes in the developing brain. Many previous studies have characterized the neural oscillatory responses serving movement, which include a beta event-related desynchronization (ERD) preceding movement onset, gamma and theta responses coinciding with movement execution, and a post-movement beta-rebound (PMBR) response following movement offset. While a few studies have investigated the developmental trajectories of these neural oscillations serving motor control, the impact of pubertal hormone levels on the maturation of these dynamics has not yet been examined. Since the timing and tempo of puberty varies greatly between individuals, pubertal hormones may uniquely impact the maturation of motor cortical oscillations distinct from other developmental metrics, such as age. In the current study we quantified these oscillations using magnetoencephalography (MEG) and utilized chronological age and measures of endogenous testosterone as indices of development during the transition from childhood to adolescence in 69 youths. Mediation analyses revealed complex maturation patterns for the beta ERD, in which testosterone predicted both spontaneous baseline and ERD power through direct and indirect effects. Age, but not pubertal hormones, predicted motor-related theta, and no relationships between oscillatory responses and developmental metrics were found for gamma or PMBR responses. These findings provide novel insight into how pubertal hormones affect motor-related oscillations, and highlight the continued development of motor cortical dynamics throughout the pubertal period.
Keywords: Beta, Puberty, Magnetoencephalography, Motor, Hormones
1. Introduction
The pubertal period of development consists of many physiological changes, including increased production of the sex steroids testosterone and estradiol, which facilitate sex-specific developmental processes (Sisk and Foster, 2004). These hormones induce molecular and cellular processes such as neurogenesis, cell survival, synaptic pruning, and programmed cell death to influence cortical structural changes (Ahmed et al., 2008; Davis et al., 1996; Nuñez et al., 2002), which are thought to result in sexually dimorphic patterns of grey and white matter development (De Bellis, 2001; Giedd et al., 1999; Gogtay et al., 2004; Shaw et al., 2008). Of note, intracellular androgen receptors are present throughout the human cerebrum, but are particularly densely distributed within the motor and somatosensory cortices (Kritzer, 2004). Further, the structural growth trajectories of these posterior frontal and parietal regions have been associated with levels of endogenous pubertal hormones in both males and females (Bramen et al., 2012; Koolschijn et al., 2014; Neufang et al., 2009; Nguyen, 2018; Paus et al., 2010; Peper et al., 2009; Vijayakumar et al., 2018).
The sensorimotor cortices are also known to be critical for generating the neural oscillations that serve motor control, which have been extensively characterized in healthy adults. Briefly, an event-related desynchronization (ERD) within the beta range (14–30 Hz), beginning prior to movement onset and remaining active through the duration of the movement, has been consistently associated with movement planning and execution (Cheyne, 2013; Gaetz et al., 2010; Heinrichs-Graham et al., 2016, 2018b, 2020; Heinrichs-Graham and Wilson, 2015, 2016; Spooner et al., 2021b; Trevarrow et al., 2019; Wiesman et al., 2020; Wilson et al., 2010, 2014). Following this response, there is a resynchronization in the beta band, termed the post-movement beta rebound (PMBR), which is thought to be instrumental in functional inhibition of the motor system and/or sensory reafference (Fry et al., 2016; Gaetz et al., 2010; Heinrichs-Graham et al., 2017; Jurkiewicz et al., 2006; Wilson et al., 2011a,b). Other motor-related oscillatory responses include the movement-related gamma (>30 Hz) synchronization (MRGS) and theta (3–6 Hz) synchronizations that occur at movement onset and are thought to index motor execution and higher order processing (Cheyne et al., 2008; Heinrichs-Graham et al., 2018a; Karakaş, 2020; Muthukumaraswamy, 2010; Spooner et al., 2021c, 2021a, 2020; Wiesman et al., 2021, 2020; Wilson et al., 2010).
Major neurophysiological changes are known to occur in the motor cortices across the lifespan (Heinrichs-Graham et al., 2018b; Heinrichs-Graham and Wilson, 2016; Rossiter et al., 2014; Spooner et al., 2021a), and there is a growing wealth of knowledge documenting developmental changes in the neural oscillatory dynamics serving motor control in youth (Gaetz et al., 2010; Heinrichs-Graham et al., 2020, 2018b; Kurz et al., 2016; Trevarrow et al., 2019; Wilson et al., 2010). Importantly, these studies have only used chronological age as a metric for development. Given the known impact of pubertal hormones on cortical structure in somato-motor areas, and the vast individual variability in the timing and tempo of pubertal maturation (Blakemore et al., 2010; Sisk and Foster, 2004), there is a critical need for studies that disentangle chronological age and pubertal effects on cortical maturation, especially within the domain of motor control.
To date, most neuroimaging studies investigating pubertal hormone changes have largely focused on the maturation of social and affective processes using fMRI (for review, see (Vijayakumar et al., 2018)). Far fewer studies have examined how hormones influence neural oscillations serving cognitive performance (Fung et al., 2020, Fung et al., 2022) and any divergence from age-specific effects on oscillatory brain development (Embury et al., 2019; Fung et al., 2021; Killanin et al., 2020; Ott et al., 2021; Taylor et al., 2021, 2020). Thus, pubertal hormones may also uniquely impact the development of motor cortical oscillations distinct from other developmental metrics like chronological age. In the present study, we use magnetoencephalography (MEG) to study the development of volitional motor responses in relation to endogenous pubertal testosterone levels in a group of typically-developing children and adolescents. We focused on testosterone levels in this study because this hormone has been tightly linked to pubertal maturational changes in the body and brain. Briefly, testosterone levels sharply and consistently increase across the pubertal transition and enact developmental effects distinct from other sex steroids, therefore, rising levels of testosterone can independently serve as a reliable metric of pubertal maturation in both males and females (Shirtcliff et al., 2009). To our knowledge, no previous study has examined the impact of pubertal hormones on the developmental trajectory of motor cortical oscillations, or probed how such effects may differ from the well-described age-related changes. All participants completed the motor task during MEG and the resulting neural responses were transformed into the time-frequency domain and imaged using a beamforming approach to identify the cortical origin of significant oscillatory motor responses. Virtual sensor time series were then extracted from the peak voxel of each response, and these were subjected to mediation analyses to identify the relationships between developmental metrics (i.e., testosterone levels and age) and oscillatory motor responses. Based on the existing literature demonstrating unique effects of hormones on structural brain maturation and age-related changes in oscillatory motor development, we hypothesized that testosterone levels would be coupled to neural oscillatory responses in the motor cortex, above-and-beyond the effects of chronological age alone.
2. Materials and methods
2.1. Participants
Seventy-four typically developing children and adolescents ages 9–15 years (Mage = 12.99 years, SD = 1.72; 41 females; 70 right-handed) completed a basic sensory detection task and provided saliva samples as part of the National Science Foundation-funded Developmental Chronnecto-Genomics (Dev-CoG) study (Stephen et al., 2021). All participants were recruited from the Omaha site. Of note, the raw MEG data from this sample of participants has been previously published (Fung et. al., 2021), though the present manuscript utilized a different index of development (i.e., hormones) and a complete reanalysis of all MEG data to probe a different functional construct (i.e., motor performance vs. visual processing in the previous paper). Exclusionary criteria, determined by parent report, included attention-deficit/hyperactivity disorder (ADHD) or other psychiatric or neurological disorders affecting brain function, history of head trauma, and general MEG/MRI exclusionary criteria such as the presence of metal implants, dental braces or permanent retainers, or other metallic or otherwise magnetic non-removable devices. All procedures were approved by the Institutional Review Board, and informed consent from the child’s parent or legal guardian, as well as assent from the child, were obtained before proceeding with the study.
2.2. Procedure
2.2.1. Salivary testosterone collection and measurement
At least 2.0 ml of whole unstimulated saliva was collected from each participant. Specifically, children were asked to passively drool into an Oragene DISCOVER (OGR-500; www.dnagenotek.com) collection tube until liquid saliva (not bubble) exceeded the fill line indicated on the tube. A single-channel pipette was then used to extract 0.5 ml from the collection tube (prior to the release of the protease inhibitors for long-term storage), and this 0.5 ml was immediately transferred into a labeled micro-centrifuge tube and placed in a −20°C freezer for storage. Participants were instructed to refrain from consuming any food, liquids, or chewing gum for at least an hour before providing the saliva sample, and samples were generally collected in the afternoon to minimize circadian influences. All samples were assayed in duplicate at the University of Nebraska Lincoln Salivary Biosciences Laboratory using a commercially-available assay kit for salivary testosterone (Salimetrics; www.salimetrics.com). The testosterone assay kit had a sensitivity of 1pg/mL, with a range of 6.1–600 pg/mL. The intra- and inter-assay coefficients of variation were 5.28% and 8.93%, respectively. The average of the duplicate tests (per hormone) were used for further analyses in the present study.
2.2.2. Task paradigm
To elicit volitional motor responses, a simple sensory detection task was performed during the MEG recording (Fig. 1; see (Fung et al., 2021; Trevarrow et al., 2019)). During this task, participants were told to fixate on a centrally presented crosshair. After a fixation period of 2400–2600 ms, one of three stimuli was presented for 800 ms: a stationary visual grating stimulus, an auditory steady-state stimulus, or both. All stimuli were supra-threshold and easily detected. Participants were asked to respond with a button press using their right index finger when any stimulus was detected. Each participant performed 300 trials (100 of each condition) in a pseudo-randomized order. The total MEG task duration was 16.5 minutes. Responses with reaction times that were 2.5 SDs above or below the participant’s mean were excluded from the final analyses. Of note, given our goals and hypotheses, the present study focused on the 100 visual-only trials to minimize the spatial overlap between primary sensory and motor responses, maximize the homogeneity of the neural responses, and ensure that the longer duration auditory steady-state responses (Wilson et al., 2012, 2008, 2007) did not bias the later motor-related oscillations.
Fig. 1.
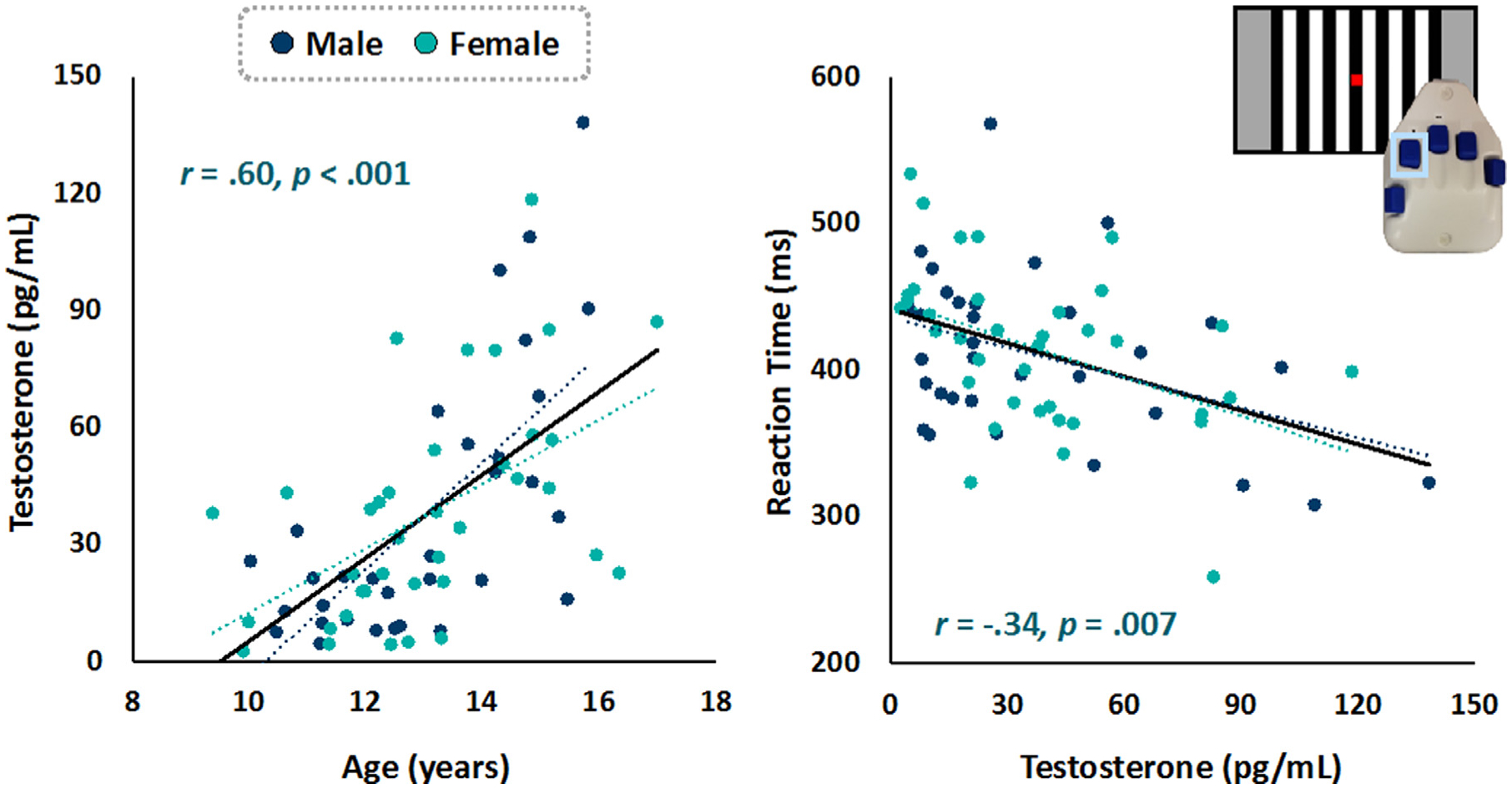
Hormone and Behavioral Results. (Left) Scatterplot shows chronological age was positively correlated with testosterone, such that older youth had higher levels of endogenous testosterone. (Right) The sensory detection task paradigm consisted of a visually-presented grating stimulus. Participants were instructed to push a button when the stimulus was detected. The scatterplot shows that more mature children with higher testosterone levels performed better on the task (i.e., had faster reaction times). This effect remained significant when controlling for age (r = −.31, p = .01). Note that males and females are plotted separately, though we did not detect significant sex differences in either correlation.
2.2.3. MEG data acquisition
MEG recordings were conducted in a one-layer magnetically shielded room with active shielding engaged. Neuromagnetic responses were acquired with a MEGIN MEG system with 306 magnetic sensors (204 planar gradiometers, 102 magnetometers; MEGIN, Helsinki, Finland) using a bandwidth of 0.1–330 Hz, sampled continuously at 1 kHz. Each participant’s data were individually corrected for head motion, and noise reduction was applied using the signal space separation method with a temporal extension (tSSS; (Taulu et al., 2005; Taulu and Simola, 2006)).
2.2.4. MEG Coregistration and Structural MRI processing
Preceding MEG measurement, four coils were attached to the participant’s head and localized, together with the three fiducial points and scalp surface, using a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the MEG sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system, each participant’s MEG data were coregistered with their individual structural T1-weighted MRI data prior to source space analyses using BESA MRI (Version 2.0). Structural T1-weighted MRI images were acquired using a Siemens Skyra 3T MRI scanner with a 32-channel head coil and a MP-RAGE sequence with the following parameters: TR = 2400 ms; TE = 1.94 ms; flip angle = 8°; FOV = 256 mm; slice thickness = 1 mm (no gap); voxel size = 1 × 1 × 1 mm. MRI, MEG, and assessment data were captured via the collaborative informatic and neuroimaging suite (COINS; (Scott et al., 2011)). These data were aligned parallel to the anterior and posterior commissures and transformed into standardized space. Following source reconstruction (i.e., beamforming), each participant’s 4.0 × 4.0 × 4.0 mm functional images were also transformed into standardized space using the transform that was previously applied to the structural MRI volume and spatially resampled.
2.2.5. MEG time-frequency transformation and statistics
Cardiac and ocular artifacts were removed from the data using signal-space projection (SSP), which was accounted for during source reconstruction (Uusitalo and Ilmoniemi, 1997). The continuous magnetic time series was divided into epochs of 2200 ms duration, with a baseline extending from −1200 to −800 ms before movement onset, which was defined as 0 ms. Epochs containing artifacts (e.g., eye blinks, muscle artifacts, eye saccades, swallowing, coughing) were rejected based on a fixed-threshold method, supplemented with visual inspection. Briefly, the distribution of amplitude and gradient values per participant were computed using all trials, and the highest amplitude/gradient trials relative to the total distribution were excluded by selecting a threshold that rejected extreme values. Notably, thresholds were set independently for each participant due to differences among individuals in head size and sensor proximity, which strongly affect MEG signal amplitude. An average amplitude threshold of 1329 (SD = 307) and an average gradient threshold of 510 (SD = 204) in the sample were identified. After artifact rejection, an average of 85.5 (SD = 4.7) trials per participant were used for further analysis, and this number was not significantly correlated with chronological age (r = .01, p = .94) or testosterone (r = .06, p = .64).
Artifact-free epochs were transformed into the time-frequency domain using complex demodulation (Kovach and Gander, 2016; Papp and Ktonas, 1977), and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized using the respective bin’s baseline power, which was calculated as the mean power during the −1200 to −800 ms time period. The specific time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. To reduce the risk of false-positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error. In the first stage, two-tailed paired-sample t-tests against baseline were conducted on each data point, and the output spectrograms of t-values were thresholded at p < .05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, the time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also below the p < .05 threshold, and a cluster value was derived by summing the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values and the significance level of the observed clusters (from stage one) was tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, 1,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time-frequency windows that contained significant oscillatory events across all participants were subjected to a beamforming analysis (see Results).
2.2.6. MEG source imaging, virtual sensor analyses, and statistics
Cortical responses were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001; Hillebrand et al., 2005; Van Veen et al., 1997), which employs spatial filters in the time-frequency domain to calculate source power for the entire brain volume. The single images were derived from the cross-spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, we computed noise-normalized, source power per voxel in each participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth (Hillebrand et al., 2005). Such images are typically referred to as pseudo-t maps, with units (i.e., pseudo-t) that reflect noise-normalized power differences (i.e., active vs. passive) per voxel. MEG preprocessing and imaging used the BESA (V 6.1) software.
Normalized differential source power was computed for the statistically-selected time-frequency bands (see below) over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. The resulting 3D maps of brain activity were then averaged across participants to assess the neuroanatomical basis of the significant oscillatory responses identified through the sensor-level analyses. For each significant response, virtual sensor data were extracted from the peak voxel, which corresponded to left primary motor cortex for all time-frequency windows. To create the virtual sensors, we applied the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which yielded two time series for each coordinate in source space, and then computed the vector sum of the two orientations ultimately yielding one time series per voxel per participant (Cheyne et al., 2006). The relative power (i.e., baseline-corrected) envelope was computed for the frequency bin corresponding to the derived peak using the virtual sensor time series, as well as the absolute power (i.e., not baseline-corrected) for the corresponding baseline interval to estimate the power of spontaneous activity. Amplitude values with the time window used to compute beamformer images were then averaged per oscillatory response. These response metrics were then subjected to mediation analyses to determine whether these MEG outcome variables were related to the developmental metrics. Specifically, we explored the degree to which pubertal testosterone levels mediate the effects of chronological age on spontaneous and relative motor oscillatory dynamics, which are well-established in the literature. For a more comprehensive perspective, we included the multiple mediation of age → testosterone → baseline power → relative power for all oscillatory responses, which is likely more representative of the complete active neural system (in comparison to examining either the baseline or relative power effects in isolation of one another). We utilized bias-corrected confidence intervals based on 1,000 bootstrapped samples to more robustly detect any potential relationships between brain activity and development (Austin and Tu, 2004; Efron and Tibshirani, 1986; Fritz and MacKinnon, 2007). Mediation analyses were conducted in Mplus (version 8.1).
3. Results
3.1. Demographic and hormone results
Of the 74 participants who completed the task, five were excluded due to excessively noisy MEG data or other errors in MEG data acquisition. Thus, the final sample consisted of 69 children and adolescents (Mage = 13.05 years, SD = 1.74; 37 females; 65 right-handed).
As expected, chronological age was positively correlated with testosterone levels in the whole sample (r = .60, p < .001; Fig. 1), such that older children tended to have higher levels of testosterone. We did not detect any significant sex difference in the correlation between age and testosterone levels.
3.2. Behavioral results
Participants performed well on the sensory detection task, responding to 97.03 ± 3.97% of trials with an average reaction time of 395.25 ± 55.10 ms. There was a significant negative correlation between age and reaction time (r = −.33, p = .006), such that older participants had faster reaction times. A negative association between testosterone and reaction time (r = −.34, p = .007; Fig. 1) was also observed and remained significant when controlling for chronological age (r = −.31, p = .01). There were no sex differences in performance, and the relationship between testosterone and reaction time did not statistically differ between males and females.
3.3. Neural oscillatory responses to the task
Statistical analysis of the time-frequency spectrograms showed significant oscillatory responses in four distinct time-frequency windows (all p-values < .001 after cluster-based permutation testing) across sensors near the sensorimotor cortices (Fig. 2). These corresponded to the widely known motor-related oscillations and included a significant decrease from baseline levels (i.e., desynchronization) in the beta range (18–26 Hz; beta ERD) that started −150 ms before movement onset and persisted 250 ms afterward. A significant increase from baseline beta levels (i.e., synchronization) after the movement was completed from 600–900 ms (16–22 Hz; PMBR), as well as synchronizations in the theta (3–6 Hz, −200 to 150 ms) and gamma (72–84 Hz, −50 to 75 ms; MRGS) ranges (all ps < .001, corrected). These four significant oscillatory responses were imaged using a beamformer, and the resulting maps were grand-averaged per oscillatory response. All four responses were tightly clustered on the left precentral gyrus, corresponding to the primary motor cortex (i.e., contralateral to a button press of the right index finger).
Fig. 2.
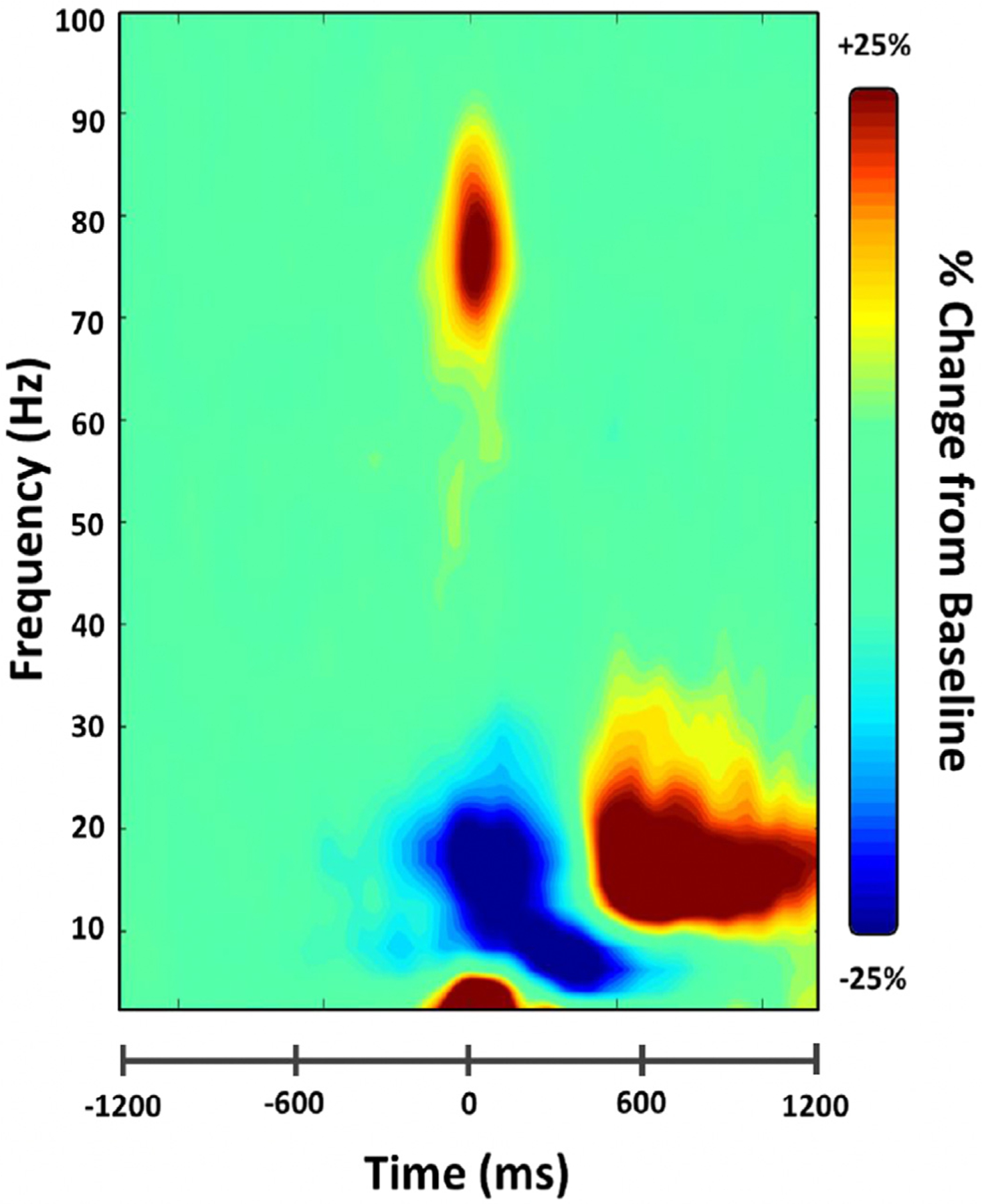
Time-frequency spectogram of motor responses. Data are displayed from a representative sensor near the left sensorimotor cortices and have been averaged across all participants. Warm colors reflect power increases relative to the baseline (i.e., synchronizations), and cool colors represent decreases relative to baseline (desynchronizations). Zero ms denotes movement onset. Time frequency windows for source imaging (beamforming) were derived from statistical analyses of these sensor-level spectrograms, which indicated significant bins in theta, beta, and gamma activity.
3.4. Beta ERD response
Mediation analyses were performed to examine the relationship between development and neural oscillatory responses, which revealed that testosterone mediated the effect of age on beta ERD response metrics in the primary motor cortex (Fig. 3). Specifically, age was significantly associated with testosterone levels (β = .66, p < .001), and testosterone was related to the strength of the beta ERD response (β = .32, p = .037). Spontaneous beta power during the baseline period also predicted the strength of this subsequent beta ERD (β = −.35, p = .003), with higher spontaneous beta power predicting stronger (i.e., more negative) beta desynchronization. Further, there was a significant indirect effect of age on the strength of the beta ERD response via testosterone levels (β = .21, b = .002, CI [.005, .041]), such that older age predicted higher testosterone values, which ultimately predicted weaker oscillatory beta ERD responses (i.e., negative values closer to zero, or weaker desynchronization). There was also a significant indirect effect of age on spontaneous beta power via testosterone (β = −.17, b = −2.49, CI [−5.92, −.15]), whereby greater age predicted more testosterone, which subsequently predicted weaker spontaneous beta activity during the baseline. The direct effects of age on beta ERD power (β = −.21, p = .17) and baseline beta power (β = .08, p = .65) were not significant; thus, the model suggests that these beta oscillatory metrics were fully mediated by testosterone levels.
Fig. 3.
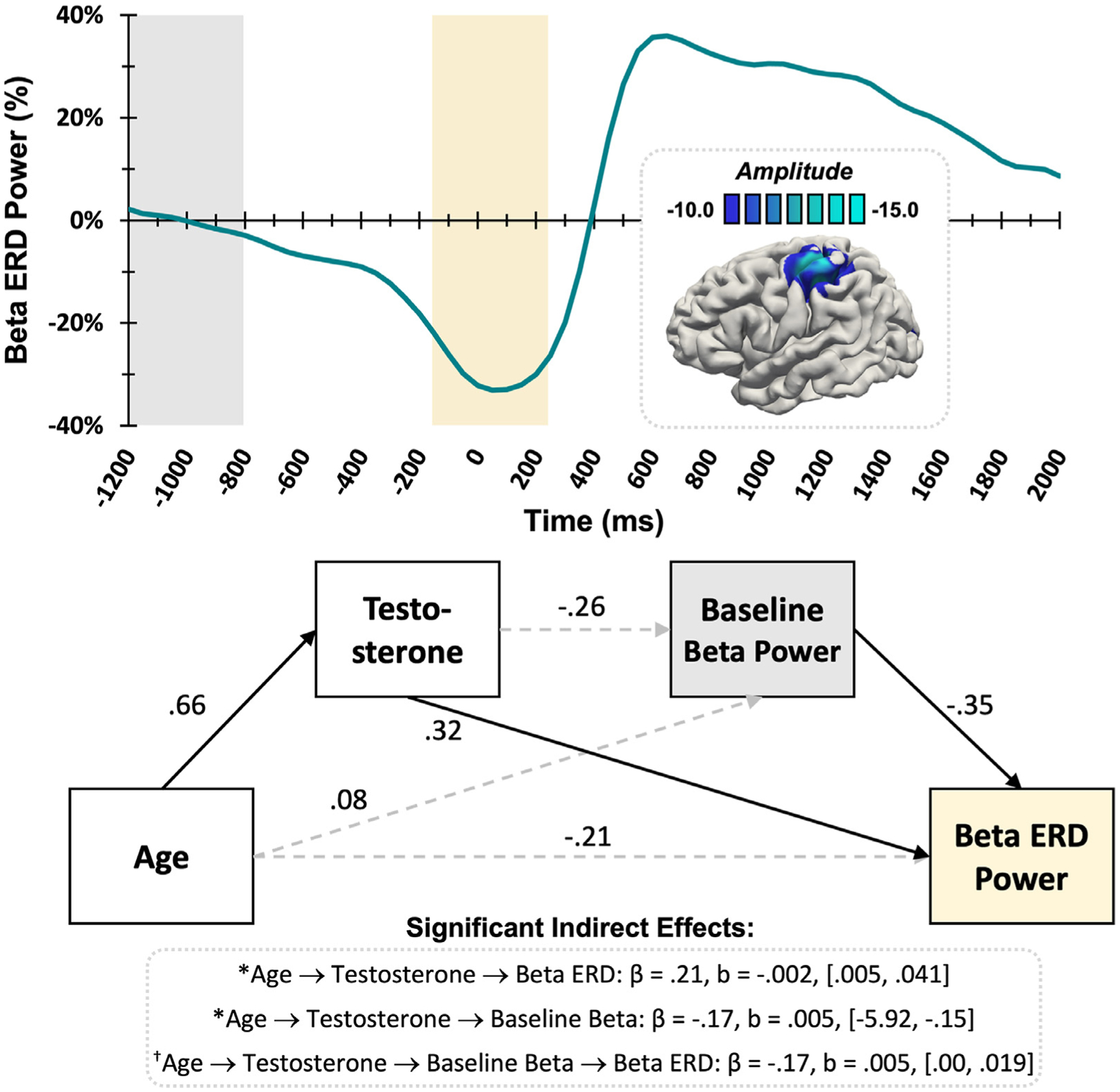
Beta ERD Response. (Top) The superimposed brain image shows that the average beta ERD response across the entire sample was centered on the contralateral primary motor cortex near the motor hand-knob feature. The color scale bar in pseudo-t units appears above the image. The time series envelope depicts changes in power relative to the baseline at the peak voxel in the beta range (18–26 Hz). The yellow box indicates the time window that was significantly different from the baseline period (grey box) based on the MEG sensor-level analyses. (Bottom) The statistical diagram demonstrates the results of the mediation analyses for the beta ERD response, with standardized coefficients shown. Direct effects are displayed graphically, where solid lines signify statistically significant relationships at p < .05 and dashed lines show nonsignificant relationships, while significant indirect effects are printed below. Testosterone significantly mediated age-related changes in the strength of both spontaneous beta activity during the baseline and beta ERD power. A full mediation was trending towards significance, where older age predicted higher testosterone, lower spontaneous beta power, and lastly weaker (i.e., less negative) beta ERD response power. * = significant, † = trending
Of note, the full mediation with age predicting testosterone, predicting baseline spontaneous beta power, predicting beta ERD power was approaching significance (β = −.17, b = .005, CI [.00, .019]). The direct effect of age on beta ERD power was not significant (β = −.21, p = .17), thus the model suggests this relationship would be fully mediated by testosterone and spontaneous beta power during the baseline in a larger (better-powered) sample.
3.5. PMBR response
The average post-movement beta rebound (PMBR) response was centered on the contralateral primary motor cortex (Fig. 4A). Age was not directly related to PMBR power with the inclusion of other mediating factors in the model, though this relationship was approaching significance (β = .30, p = .071). There were no other significant associations where developmental metrics predicted baseline or PMBR response power.
Fig. 4.
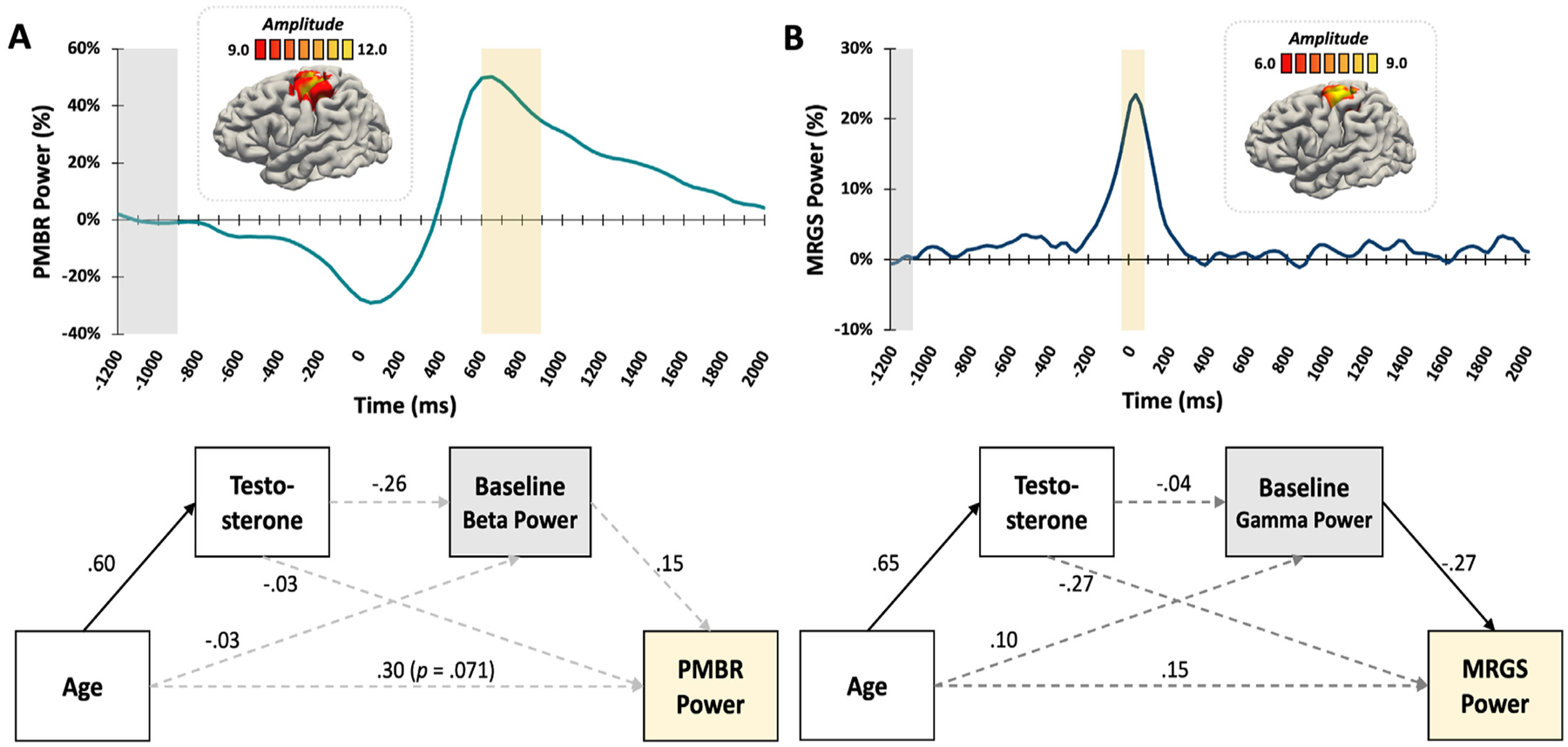
PMBR and MRGS Responses. (Top) Brain images show that (A) the PMBR response and (B) the peak gamma response (MRGS) were both strongest in the left primary motor cortex. Color scale bar in pseudo-t units appears above each image. The time series envelopes (PMBR: 16–22 Hz; MRGS: 72–84 Hz) depict changes in power relative to the baseline at the peak voxels of each response. The yellow box indicates the time window that was significantly different from the baseline period (grey box) based on the MEG sensor-level analyses. (Bottom, A) Results of the mediation model for the PMBR response showed that older age predicted higher testosterone, but there were no further significant assocations. (Bottom, B) Results of the mediation model for the MRGS indicated that older age predicted more testosterone, and higher spontaneous gamma during the baseline predicted weaker MRGS responses. No further mediation effects were significant. Solid lines signify statistically significant relationships at p < .05, dotted lines represent nonsignificant relationships. All reported coefficients are standardized.
3.6. Movement-related Gamma Synchronization (MRGS) response
There were two significant effects for the MRGS response in the primary motor cortex (Fig. 4B). Age was positively associated with testosterone (β = .72, p < .001) and spontaneous gamma power during the baseline was inversely related to MRGS power (β = −.47, p = .047), such that increased age predicted increased testosterone, and higher spontaneous baseline gamma predicted weaker MRGS response power, respectively. However, we did not detect any indirect effects or relationships between developmental metrics and gamma response variables.
3.7. Movement-related Theta ERS responses
Statistical analyses revealed that age directly predicted both testosterone (β = .72, p < .001) and spontaneous theta power during the baseline (β = −.45, p = .014) in the motor cortex (Fig. 5), with the latter decreasing with increasing age. Subsequently, stronger baseline spontaneous theta power was associated with weaker theta ERS responses (β = −.47, p = .002). The relationship between age and theta ERS power was fully mediated by spontaneous theta power during the baseline (β = .21, b = .05, CI [.01, .10]), as the direct relationship between age and theta ERS power was not significant (β = −.02, p = .93). Thus, older youth had weaker baseline theta activity and subsequently stronger theta ERS responses.
Fig. 5.
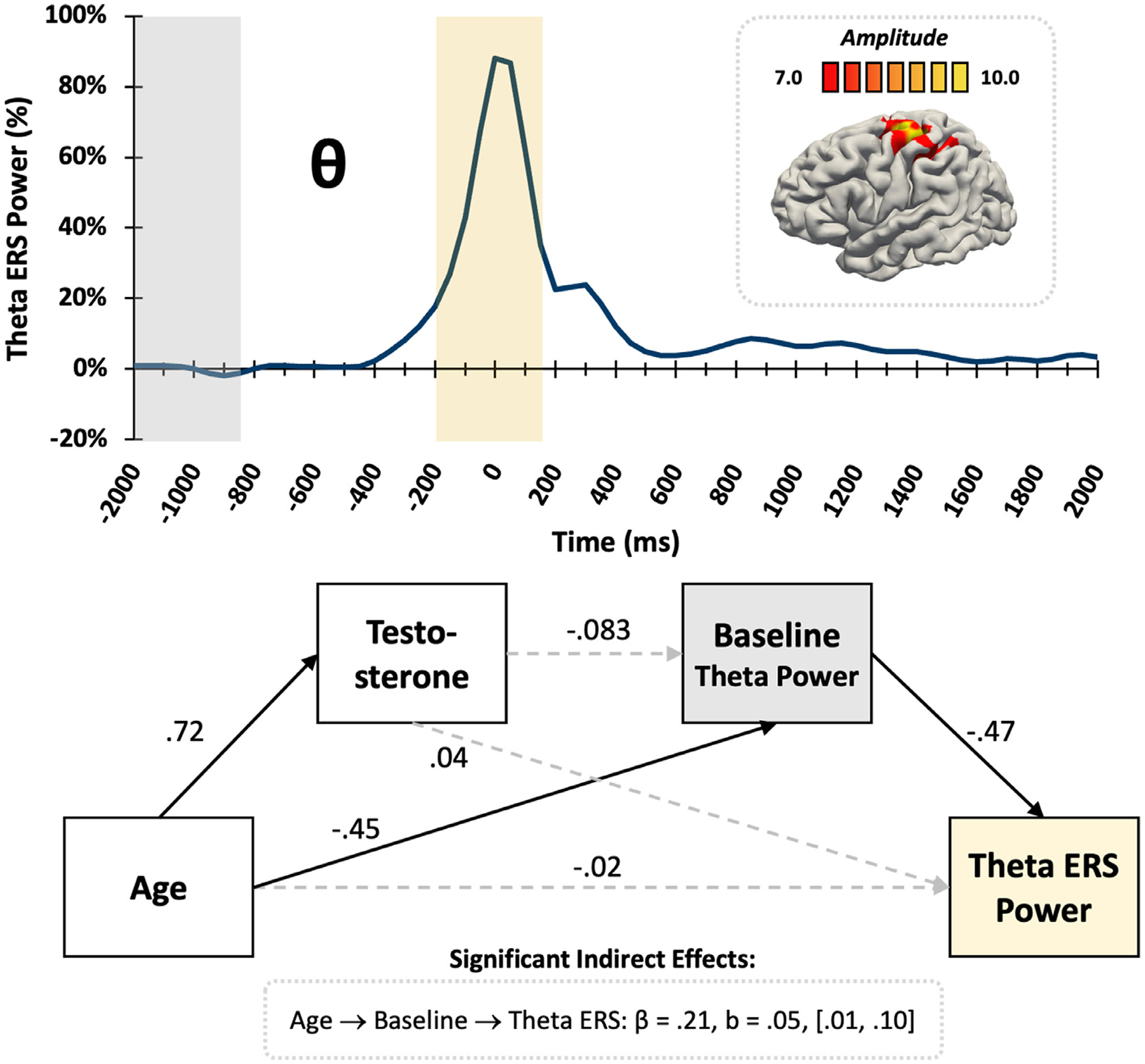
Motor-related theta ERS. (Top) The average brain map shows the theta ERS was strongest in the left motor cortex, with the color scale bar in pseudo-t units directly above. The theta time series envelope (3–6 Hz) extracted from the peak voxel shows a significant increase in theta power (i.e., ERS; yellow box) around movement onset relative to the baseline period (grey box). (Bottom) The statistical diagram demonstrates the partial mediation of theta response metrics, with standardized coefficients shown. Solid lines signify statistically significant relationships at p < .05, dotted lines represent nonsignificant relationships. The indirect effect of age through spontaneous theta power during the baseline significantly predicted the strength of the theta ERS response.
4. Discussion
In this study, we examined the developmental trajectory of neural oscillatory activity serving basic motor control in relation to pubertal testosterone levels in a sample of typically-developing youth. We observed four well known motor-related responses, including the peri-movement beta ERD, the PMBR, and the MRGS and theta ERS during movement execution. All responses localized to the contralateral primary motor cortex, corroborating prior MEG studies of motor-related neural oscillations in youths and adults (Cheyne, 2013; Gaetz et al., 2010; Heinrichs-Graham et al., 2020, 2018a, 2018b, 2017, 2016; Heinrichs-Graham and Wilson, 2016, 2015; Kurz et al., 2016; Spooner et al., 2021b; Trevarrow et al., 2019; Wiesman et al., 2021, 2020; Wilson et al., 2014, 2010). Importantly, we found novel developmental shifts in these oscillatory responses in terms of chronological age and pubertal-specific effects, as indexed by testosterone levels. Mediation analyses revealed complex maturation patterns for the beta ERD response, in which testosterone predicted both spontaneous beta levels and the beta ERD response through direct and indirect effects. Age, but not pubertal hormones, predicted motor-related theta ERS activity, and no relationships between oscillatory outcomes and developmental metrics were found for the PMBR and MRGS responses. Below, we discuss the implications of these findings for understanding the developmental trajectory of cortical motor oscillations during the pubertal transition.
Our most interesting findings were the complex mediation effects of the developmental metrics on peri-movement beta activity. Consistent with previous studies (Heinrichs-Graham et al., 2018b; Heinrichs-Graham and Wilson, 2016; Wilson et al., 2014), participants with the strongest spontaneous beta activity during the baseline also had the strongest beta ERD (i.e., most negative) prior to and during movement, with both direct and indirect effects. Importantly, the mediation analyses indicated the influence of endogenous testosterone levels on these beta oscillations, as higher testosterone was predictive of weaker spontaneous beta activity during the baseline. These findings corroborate prior work showing spontaneous beta power decreases as youth enter later adolescence and early adulthood, potentially indicative of “optimized” resting motor cortices and enhanced neural efficiency (Heinrichs-Graham et al., 2018b). Thus, our mediation analyses contextualize prior literature focused on the effects of chronological age on motor-related oscillations, and show that the effect of testosterone explains the effect of age on beta oscillatory activity, at least to some degree. Beta oscillatory activity is largely dependent on GABA-mediated local inhibitory networks, especially within the primary motor cortices (Hall et al., 2011; Yamawaki et al., 2008). This may suggest a key role of pubertal sex-steroids in the development of these GABA-mediated beta oscillatory responses. The maturation of local GABAergic circuitry continues into adolescence and adulthood (Hashimoto et al., 2009; Kilb, 2012), and studies have shown that sex steroids can influence GABA subunit expression and modulate GABAergic activity (Belelli et al., 2006; Belelli and Lambert, 2005; Fries et al., 2007; Zhang et al., 1999). Previous studies that pharmacologically manipulated the GABA system in human adults observed subsequent changes in baseline spontaneous beta power and beta ERD responses (Muthukumaraswamy et al., 2013). We found that testosterone mediated both spontaneous beta and subsequent beta ERD responses during movement, which supports prior findings of both spontaneous and active beta oscillatory responses being influenced by GABAergic drive (Hall et al., 2011; Muthukumaraswamy et al., 2013). Therefore, the pubertal rise in androgenic hormones and other sex steroids likely contributes to the development of local GABAergic networks in the motor cortices that facilitate changes in the beta oscillatory activity serving motor control.
Interestingly, our findings did not suggest any significant mediation effects of age or testosterone on the PMBR or MRGS responses. Thus, testosterone did not appear to modulate the development of these responses. Prior work has shown that the PMBR and MRGS responses in the primary motor cortices are more GABA-independent (Hall et al., 2011), which may suggest that they would be less affected by the impact of changing pubertal hormone levels on the development of GABA-inhibitory circuits. Of note, our data indicated a trending direct effect of age predicting PMBR power, which would have be consistent with previous studies that used different motor tasks in smaller developmental samples (Gaetz et al., 2010; Trevarrow et al., 2019; Wilson et al., 2010). Since oscillatory motor activity is largely modulated by movement characteristics such as the certainty and complexity of movement (Gaetz et al., 2013; Grent-’t-Jong et al., 2013; Heinrichs-Graham et al., 2018a, 2016; Heinrichs-Graham and Wilson, 2015; Kaiser et al., 2001; Tan et al., 2016; Tzagarakis et al., 2015, 2010; Wiesman et al., 2021, 2020), future studies using more complex and/or cognitively-involved motor tasks may provide clarity into whether the PMBR and MRGS also show pubertal maturational effects under more demanding movement conditions, as the current study was well powered but also involved a relatively simple motor task.
In regard to motor-related theta activity, our mediation analysis showed that chronological age predicted spontaneous theta during the baseline, which subsequently predicted theta ERS, observed through significant direct and indirect effects. Of note, testosterone was not a significant predictor of oscillatory theta power within our mediation models, thus the development of motor-related theta activity likely occurs through a different mechanism distinct from the influence of testosterone. Future studies should further investigate other potential developmental markers, such as DHEA, to determine the main contributors to the development of motor-related theta oscillations.
Before closing, it is important to acknowledge some limitations of the present study. Circulating hormones, such as testosterone, are cyclic in nature and inherently fluctuate between individuals on the bases of sex, time of day, and other situational factors (Herting and Sowell, 2017; Matchock et al., 2007). In the present study, salivary samples were collected at similar times and sample concentrations did not differ by sex, thus these fluctuations potentially had minor effects on our data that were largely accounted for. Nonetheless, future work using alternative hormonal assay methods, such as hair hormone concentrations, could provide a more representative, average level of physiologically active pubertal hormones (Short et al., 2016). Future studies should also investigate the influence of other sex steroids, such as DHEA, progesterone, and estradiol, which dramatically change throughout pubertal development and have the potential to influence other components involved in the maturation of neurophysiological responses and cortical systems. Though the present study utilized a substantial sample size (n = 74), our data was potentially underpowered to detect more subtle patterns of oscillatory development. Specifically, the full mediation of the beta ERD response (age → testosterone → baseline beta power → beta ERD) was trending towards significance, but did not surpass the threshold. Replicating this study in a larger sample would likely confirm these findings and detect other more subtle effects. Lastly, future studies should examine the effect of handedness on motor-related oscillations in developmental samples. We collapsed across this variable due to the limited number of left-handed participants, but differences in the neural dynamics serving left- and right-handed movements have been demonstrated, and developmental studies are warranted (Wiesman et al., 2021).
In conclusion, the current study was the first to investigate the influence of pubertal testosterone levels on the development of cortical oscillations serving motor control in typically-developing children and adolescents. Complex maturation patterns for the beta ERD response were observed, which reflect the major impact of testosterone changes on the development of cortical motor function during the pubertal period. Age-related changes in the theta motor response were also identified, and no significant developmental effects were observed for the PMBR and gamma ERS responses. Overall, these findings highlight the continued maturation of neural oscillations serving motor control and provide novel insight into the influence of pubertal hormones on functional cortical development during the pubertal transition.
Acknowledgements/Funding
This research was supported by grants R01-MH121101 (TWW), P20-GM144641 (TWW, EHG, and BKT), R01-MH116782 (TWW), and R01-EB020407 (VDC) from the National Institutes of Health, and grant #1539067 (TWW, VDC, Y-PW, and JMS) and #2112455 (VDC) from the National Science Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All salivary assays and analyses were performed at the University of Nebraska—Lincoln Salivary Bioscience Laboratory. We thank Dr Jessica L. Calvi for her insight and assistance with this aspect of the study.
Footnotes
Declarations of Interest
None.
Credit authorship contribution statement
Madison H. Fung: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing, Visualization. Elizabeth Heinrichs-Graham: Conceptualization, Methodology, Writing – review & editing. Brittany K. Taylor: Methodology, Writing – review & editing. Michaela R. Frenzel: Investigation, Project administration. Jacob A. Eastman: Investigation. Yu-Ping Wang: Writing – review & editing, Funding acquisition. Vince D. Calhoun: Writing – review & editing, Funding acquisition. Julia M. Stephen: Writing – review & editing, Funding acquisition. Tony W. Wilson: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition.
Data availability statement
All data that support the findings of this study are available upon reasonable request to the corresponding author (TWW). Data will be made publicly available upon study completion.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL., 2008. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci 11, 995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC, Tu JV., 2004. Bootstrap Methods for Developing Predictive Models. Am Stat 58, 131–137. doi: 10.1198/0003130043277. [DOI] [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, et al. , 2006. Neuroactive steroids and inhibitory neurotransmission: Mechanisms of action and physiological relevance. Neuroscience 138, 821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ., 2005. Neurosteroids: endogenous regulators of the GABAA receptor. Nat. Rev. Neurosci 6, 565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Blakemore S, Burnett S, Dahl RE., 2010. The role of puberty in the developing adolescent brain. Hum. Brain Mapp 31, 926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, et al. , 2012. Sex Matters during Adolescence: Testosterone-Related Cortical Thickness Maturation Differs between Boys and Girls. PLoS One 7, e33850. doi: 10.1371/journal.pone.0033850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W, 2006. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum. Brain Mapp 27, 213–229. doi: 10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC., 2008. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage 42, 332–342. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- Cheyne DO., 2013. MEG studies of sensorimotor rhythms: A review. Exp. Neurol 245, 27–39. doi: 10.1016/j.expneurol.2012.08.030. [DOI] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA., 1996. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 734, 10–18. doi: 10.1016/0006-8993(96)00298-3. [DOI] [PubMed] [Google Scholar]
- De Bellis MD., 2001. Sex Differences in Brain Maturation during Childhood and Adolescence. Cereb. Cortex 11, 552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R, 1986. Bootstrap Methods for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Stat Sci 1, 54–75. doi: 10.1214/ss/1177013815. [DOI] [Google Scholar]
- Embury CM, Wiesman AI, Proskovec AL, Mills MS, Heinrichs-Graham E, Wang Y-P, et al. , 2019. Neural dynamics of verbal working memory processing in children and adolescents. Neuroimage 185, 191–197. doi: 10.1016/j.neuroimage.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MD., 2004. Permutation Methods: A Basis for Exact Inference. Stat. Sci 19. doi: 10.1214/088342304000000396. [DOI] [Google Scholar]
- Fries P, Nikolić D, Singer W, 2007. The gamma cycle. Trends Neurosci. 30, 309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Fritz MS, MacKinnon DP. Required Sample Size to Detect the Mediated Effect 2007. 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry A, Mullinger KJ, O’Neill GC, Barratt EL, Morris PG, Bauer M, et al. , 2016. Modulation of post-movement beta rebound by contraction force and rate of force development. Hum. Brain Mapp 37, 2493–2511. doi: 10.1002/hbm.23189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung MH, Rahman RL, Taylor BK, Frenzel MR, Eastman JA, Wang Y-P, Calhoun VD, Stephen JM, Wison TW, 2022. The impact of pubertal DHEA on the development of visuospatial oscillatory dynamics. Hum. Brain Mapp 1–13. doi: 10.1002/hbm.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung MH, Taylor BK, Frenzel MR, Eastman JA, Wang Y-P, Calhoun VD, et al. , 2020. Pubertal Testosterone Tracks the Developmental Trajectory of Neural Oscillatory Activity Serving Visuospatial Processing. Cereb. Cortex 30, 5960–5971. doi: 10.1093/cercor/bhaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung MH, Taylor BK, Lew BJ, Frenzel MR, Eastman JA, Wang Y-P, et al. , 2021. Sexually dimorphic development in the cortical oscillatory dynamics serving early visual processing. Dev. Cogn. Neurosci 50, 100968. doi: 10.1016/j.dcn.2021.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Liu C, Zhu H, Bloy L, Roberts TPL., 2013. Evidence for a motor gamma-band network governing response interference. Neuroimage 74, 245–253. doi: 10.1016/j.neuroimage.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, MacDonald M, Cheyne D, Snead OC., 2010. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: Age predicts post-movement beta rebound. Neuroimage 51, 792–807. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. , 1999. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci 2, 861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. , 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl Acad. Sci 101, 8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Oostenveld R, Jensen O, Medendorp WP, Praamstra P, 2013. Oscillatory dynamics of response competition in human sensorimotor cortex. Neuroimage 83, 27–34. doi: 10.1016/j.neuroimage.2013.06.051. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R, 2001. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc. Natl Acad. Sci 98, 694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SD, Stanford IM, Yamawaki N, McAllister CJ, Rönnqvist KC, Woodhall GL, et al. , 2011. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage 56, 1506–1510. doi: 10.1016/j.neuroimage.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, et al. , 2009. Protracted Developmental Trajectories of GABAA Receptor α1 and α2 Subunit Expression in Primate Prefrontal Cortex. Biol. Psychiatry 65, 1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Arpin DJ, Wilson TW., 2016. Cue-related Temporal Factors Modulate Movement-related Beta Oscillatory Activity in the Human Motor Circuit. J. Cogn. Neurosci 28, 1039–1051. doi: 10.1162/jocn_a_00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Hoburg JM, Wilson TW., 2018a. The peak frequency of motor-related gamma oscillations is modulated by response competition. Neuroimage 165, 27–34. doi: 10.1016/j.neuroimage.2017.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Kurz MJ, Gehringer JE, Wilson TW., 2017. The functional role of post-movement beta oscillations in motor termination. Brain Struct. Funct 222, 3075–3086. doi: 10.1007/s00429-017-1387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, McDermott TJ, Mills MS, Wiesman AI, Wang Y-P, Stephen JM, et al. , 2018b. The lifespan trajectory of neural oscillatory activity in the motor system. Dev. Cogn. Neurosci 30, 159–168. doi: 10.1016/j.dcn.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Taylor BK, Wang Y-P, Stephen JM, Calhoun VD, Wilson TW., 2020. Parietal Oscillatory Dynamics Mediate Developmental Improvement in Motor Performance. Cereb. Cortex 30, 6405–6414. doi: 10.1093/cercor/bhaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW., 2016. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage 134, 514–521. doi: 10.1016/j.neuroimage.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW., 2015. Coding complexity in the human motor circuit. Hum. Brain Mapp 36, 5155–5167. doi: 10.1002/hbm.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Sowell ER., 2017. Puberty and structural brain development in humans. Front. Neuroendocrinol 44, 122–137. doi: 10.1016/j.yfrne.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR., 2005. A new approach to neuroimaging with magnetoencephalography. Hum. Brain Mapp 25, 199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D, 2006. Post-movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. Neuroimage 32, 1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Birbaumer N, Lutzenberger W, 2001. Event-related beta desynchronization indicates timing of response selection in a delayed-response paradigm in humans. Neurosci. Lett 312, 149–152. doi: 10.1016/S0304-3940(01)02217-0. [DOI] [PubMed] [Google Scholar]
- Karakaş S, 2020. A review of theta oscillation and its functional correlates. Int. J. Psychophysiol 157, 82–99. doi: 10.1016/j.ijpsycho.2020.04.008. [DOI] [PubMed] [Google Scholar]
- Kilb W, 2012. Development of the GABAergic System from Birth to Adolescence. The Neuroscientist 18, 613–630. doi: 10.1177/1073858411422114. [DOI] [PubMed] [Google Scholar]
- Killanin AD, Wiesman AI, Heinrichs-Graham E, Groff BR, Frenzel MR, Eastman JA, et al. , 2020. Development and sex modulate visuospatial oscillatory dynamics in typically-developing children and adolescents. Neuroimage 221, 117192. doi: 10.1016/j.neuroimage.2020.117192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, Peper JS, Crone EA., 2014. The Influence of Sex Steroids on Structural Brain Maturation in Adolescence. PLoS One 9, e83929. doi: 10.1371/journal.pone.0083929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CK, Gander PE., 2016. The demodulated band transform. J. Neurosci. Methods 261, 135–154. doi: 10.1016/j.jneumeth.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer M, 2004. The Distribution of Immunoreactivity for Intracellular Androgen Receptors in the Cerebral Cortex of Hormonally Intact Adult Male and Female Rats: Localization in Pyramidal Neurons Making Corticocortical Connections. Cereb. Cortex 14, 268–280. doi: 10.1093/cercor/bhg127. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Proskovec AL, Gehringer JE, Becker KM, Arpin DJ, Heinrichs-Graham E, et al. , 2016. Developmental Trajectory of Beta Cortical Oscillatory Activity During a Knee Motor Task. Brain Topogr. 29, 824–833. doi: 10.1007/s10548-016-0500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R, 2007. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Matchock RL, Dorn LD, Susman EJ., 2007. Diurnal and Seasonal Cortisol, Testosterone, and DHEA Rhythms in Boys and Girls during Puberty. Chronobiol. Int 24, 969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD., 2010. Functional Properties of Human Primary Motor Cortex Gamma Oscillations. J. Neurophysiol 104, 2873–2885. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Myers JFM, Wilson SJ, Nutt DJ, Lingford-Hughes A, Singh KD, et al. , 2013. The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage 66, 36–41. doi: 10.1016/j.neuroimage.2012.10.054. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, et al. , 2009. Sex Differences and the Impact of Steroid Hormones on the Developing Human Brain. Cereb. Cortex 19, 464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nguyen T-V, 2018. Developmental effects of androgens in the human brain. J. Neuroendocrinol 30, e12486. doi: 10.1111/jne.12486. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Sodhi J, Juraska JM., 2002. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex: Sexual Differentiation of Rat Visual Cortex. J. Neurobiol 52, 312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Ott LR, Penhale SH, Taylor BK, Lew BJ, Wang Y-P, Calhoun VD, et al. , 2021. Spontaneous cortical MEG activity undergoes unique age- and sex-related changes during the transition to adolescence. Neuroimage 244, 118552. doi: 10.1016/j.neuroimage.2021.118552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp N, Ktonas P, 1977. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed. Sci. Instrum 13, 135–145. [PubMed] [Google Scholar]
- Paus T, Nawaz-Khan I, Leonard G, Perron M, Pike GB, Pitiot A, et al. , 2010. Sexual dimorphism in the adolescent brain: Role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm. Behav 57, 63–75. doi: 10.1016/j.yhbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, et al. , 2009. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology 34, 332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Rossiter HE, Davis EM, Clark EV, Boudrias M-H, Ward NS., 2014. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage 91, 360–365. doi: 10.1016/j.neuroimage.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Courtney W, Wood D, De la Garza R, Lane S, Wang R, et al. , 2011. COINS: An Innovative Informatics and Neuroimaging Tool Suite Built for Large Heterogeneous Datasets. Front. Neuroinformatics 5. doi: 10.3389/fninf.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. , 2008. Neurodevelopmental Trajectories of the Human Cerebral Cortex. J. Neurosci 28, 3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD., 2009. Pubertal Development: Correspondence Between Hormonal and Physical Development: Hormonal Correlates of Pubertal Stage. Child Dev. 80, 327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short SJ, Stalder T, Marceau K, Entringer S, Moog NK, Shirtcliff EA, et al. , 2016. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology 71, 12–18. doi: 10.1016/j.psyneuen.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL., 2004. The neural basis of puberty and adolescence. Nat. Neurosci 7, 1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Spooner RK, Arif Y, Taylor BK, Wilson TW., 2021a. Movement-Related Gamma Synchrony Differentially Predicts Behavior in the Presence of Visual Interference Across the Lifespan. Cereb. Cortex 31, 5056–5066. doi: 10.1093/cercor/bhab141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Taylor BK, Ahmad IM, Dyball KN, Emanuel K, Fox HS, et al. , 2021b. Neural oscillatory activity serving sensorimotor control is predicted by superoxide-sensitive mitochondrial redox environments. Proc. Natl Acad. Sci 118. doi: 10.1073/pnas.2104569118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Proskovec AL, Heinrichs-Graham E, Wilson TW., 2020. Prefrontal theta modulates sensorimotor gamma networks during the reorienting of attention. Hum. Brain Mapp 41, 520–529. doi: 10.1002/hbm.24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RK, Wiesman AI, Wilson TW., 2021c. Peripheral Somatosensory Entrainment Modulates the Cross-Frequency Coupling of Movement-Related Theta-Gamma Oscillations. Brain Connect doi: 10.1089/brain.2021.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen JM, Solis I, Janowich J, Stern M, Frenzel MR, Eastman JA, et al. , 2021. The Developmental Chronnecto-Genomics (Dev-CoG) study: A multimodal study on the developing brain. Neuroimage 225, 117438. doi: 10.1016/j.neuroimage.2020.117438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Wade C, Brown P, 2016. Post-Movement Beta Activity in Sensorimotor Cortex Indexes Confidence in the Estimations from Internal Models. J. Neurosci 36, 1516–1528. doi: 10.1523/JNEUROSCI.3204-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J, 2006. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol 51, 1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M, 2005. Applications of the signal space separation method. IEEE Trans. Signal Process 53, 3359–3372. doi: 10.1109/TSP.2005.853302. [DOI] [Google Scholar]
- Taylor BK, Eastman JA, Frenzel MR, Embury CM, Wang Y-P, Calhoun VD, et al. , 2021. Neural oscillations underlying selective attention follow sexually divergent developmental trajectories during adolescence. Dev. Cogn. Neurosci 49, 100961. doi: 10.1016/j.dcn.2021.100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Embury CM, Heinrichs-Graham E, Frenzel MR, Eastman JA, Wiesman AI, et al. , 2020. Neural oscillatory dynamics serving abstract reasoning reveal robust sex differences in typically-developing children and adolescents. Dev. Cogn. Neurosci 42, 100770. doi: 10.1016/j.dcn.2020.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarrow MP, Kurz MJ, McDermott TJ, Wiesman AI, Mills MS, Wang Y-P, et al. , 2019. The developmental trajectory of sensorimotor cortical oscillations. Neuroimage 184, 455–461. doi: 10.1016/j.neuroimage.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G, 2010. Beta-Band Activity during Motor Planning Reflects Response Uncertainty. J. Neurosci 30, 11270–11277. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, West S, Pellizzer G, 2015. Brain oscillatory activity during motor preparation: effect of directional uncertainty on beta, but not alpha, frequency band. Front. Neurosci 9. doi: 10.3389/fnins.2015.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ., 1997. Signal-space projection method for separating MEG or EEG into components. Med. Biol. Eng. Comput 35, 135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, Van Drongelen W, Yuchtman M, Suzuki A, 1997. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng 44, 867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH., 2018. Puberty and the human brain: Insights into adolescent development. Neurosci. Biobehav. Rev 92, 417–436. doi: 10.1016/j.neubiorev.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Christopher-Hayes NJ, Eastman JA, Heinrichs-Graham E, Wilson TW., 2021. Response certainty during bimanual movements reduces gamma oscillations in primary motor cortex. Neuroimage 224, 117448. doi: 10.1016/j.neuroimage.2020.117448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI, Koshy SM, Heinrichs-Graham E, Wilson TW., 2020. Beta and gamma oscillations index cognitive interference effects across a distributed motor network. Neuroimage 213, 116747. doi: 10.1016/j.neuroimage.2020.116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Fleischer A, Archer D, Hayasaka S, Sawaki L, 2011a. Oscillatory MEG motor activity reflects therapy-related plasticity in stroke patients. Neurorehabil. Neural Repair 25 (2), 188–193. doi: 10.1177/1545968310378511. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM., 2014. Circadian modulation of motor-related beta oscillatory responses. Neuroimage 102, 531–539. doi: 10.1016/j.neuroimage.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Hernandez OO, Asherin RM, Teale PD, Reite ML, Rojas DC., 2008. Cortical gamma generators suggest abnormal auditory circuitry in early-onset psychosis. Cereb. Cortex 18, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ., 2007. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol. Psychiatry 62, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, et al. , 2010. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 73, 75–84. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC, 2011b. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev. Neuropsychol 36 (5), 596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Wetzel MW, White ML, Knott NL., 2012. Gamma-frequency neuronal activity is diminished in adults with attention-deficit/hyperactivity disorder: a pharma-co-MEG study. J. Psychopharmacol. (Oxf) 26, 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Stanford IM, Hall SD, Woodhall GL., 2008. Pharmacologically induced and stimulus evoked rhythmic neuronal oscillatory activity in the primary motor cortex in vitro. Neuroscience 151, 386–395. doi: 10.1016/j.neuroscience.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chang YH, Feldman AN, Ma W, Lahjouji F, Barker JL, et al. , 1999. The expression of GABAA receptor α2 subunit is upregulated by testosterone in rat cerebral cortex. Neurosci. Lett 265, 25–28. doi: 10.1016/S0304-3940(99)00193-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are available upon reasonable request to the corresponding author (TWW). Data will be made publicly available upon study completion.


