Abstract
The United States Environmental Protection Agency (EPA) regulates chemical manufacture, import, processing, distribution, use, and disposal under the 2016 amended Toxic Substances Control Act (TSCA) for the purposes of protecting the public and sensitive populations—including workers—from chemical exposure risk. The publication of several TSCA risk evaluations provided a unique opportunity to evaluate the evolving regulatory approach for assessing the dermal exposure pathway in occupational settings. In this analysis, the occupational dermal exposure assessment methods employed in several TSCA risk evaluations were assessed. Specifically, a methodology review was conducted for the occupational dermal scenarios of manufacturing and feedstock use in the risk evaluations of three chlorinated organic chemicals: trichloroethylene, carbon tetrachloride, and perchloroethylene. Additionally, alternative exposure estimates were generated using the exposure model IH SkinPermTM. The review and alternative exposure analyses indicate that the current TSCA modeling approach may generate total dermal absorbed doses for chlorinated chemical manufacturing and feedstock use scenarios that are 2- to 20-fold higher than those generated by IH SkinPerm. Best-practice recommendations developed in the methodology review support a tiered, integrated approach to dermal exposure assessment that emphasizes collecting qualitative data; employing validated, peer-reviewed models that align with current industrial practices; and gathering empirical sampling data where needed. Collaboration among industry, EPA, and other stakeholders to share information and develop a standard approach to exposure assessment under TSCA would improve the methodological rigor of, and increase confidence in, the risk evaluation results.
Keywords: dermal exposure, exposure assessment, Toxic Substances Control Act, regulatory risk assessment, exposure modeling
Introduction
Chemical absorption through the skin depends on various factors, including skin condition, environmental conditions, chemical/mixture physicochemical properties, worker personal hygiene practices, protective clothing use, and general workplace conditions (Drexler, 2003; Grandjean et al., 1988). The health effects that may result from dermal exposures to chemicals in the workplace include skin irritation, skin sensitization, and systemic toxicity (Anderson and Meade, 2014; Lampel and Powell, 2019; Lushniak, 2004). The potential for systemic toxicity from skin absorption is evaluated by most U.S. and international health agencies and organizations that establish occupational exposure limits, and these agencies may assign associated occupational exposure notations (Boeniger and Ahlers, 2003; Grandjean et al., 1988; National Institute for Occupational Safety and Health, 2007; Dotson et al., 2011). In general, the resulting “skin notation” provides hazard characterization information that is used by risk managers to establish approaches to manage potential for skin contact. Thus, in most occupational scenarios at industrial facilities, dermal exposure to liquids and its subsequent risk is managed via a hazard characterization approach.
In contrast, for various chemical registration regulations in the European Union and the United States (US), a risk-based approach is often employed that includes developing a quantitative estimate of dermal dose and resulting systemic dose equivalents (European Chemicals Agency, 2016; Environmental Protection Agency, 2021a). While the US Environmental Protection Agency (EPA) evaluates occupational exposures for the new chemicals program under the Toxic Substances Control Act (TSCA), historically EPA has not evaluated or regulated occupational exposures for existing chemicals in commerce, as the Occupational Safety and Health Administration (OSHA) is at the forefront of these activities. This practice recently changed with the amendments to the TSCA. Enacted in 1976, TSCA requires EPA to protect the public from “unreasonable risk of injury to health or the environment” by regulating the manufacture, import, processing, distribution, use, and disposal of chemicals. In 2016, Congress passed the Frank R. Lautenberg Chemical Safety for the 21st Century Act, which included substantive changes to the law, including a statutory requirement to conduct human health risk evaluations for all susceptible and highly exposed populations, including workers. Consequently, EPA has had to develop a methodology for estimating occupational exposure and risk to chemical substances under specific conditions of use (COU). A COU is defined as “the circumstances, as determined by the Administrator, under which a chemical substance is intended, known, or reasonably foreseen to be manufactured, processed, distributed in commerce, used, or disposed of” (Environmental Protection Agency, 2004). If the risk evaluation indicates that a COU presents an unreasonable risk to human health or the environment, within two years of the risk evaluation being finalized, EPA must take action to mitigate the unreasonable risk.
Because of a general paucity of data for dermal exposure in US working populations, many of the TSCA risk evaluations for the first ten high-priority chemicals have relied on modeling methods to estimate dermal exposures for workers, including for three chlorinated chemicals: carbon tetrachloride, perchloroethylene, and trichloroethylene. These chemicals are volatile liquids (vapor pressures ranging from 2.5 to 58.4 kPa) and are expected to evaporate rapidly in the workplace if any liquid materials are present and open to the air. When using these chemicals as a feedstock or as an intermediate at large manufacturing facilities, daily operations typically involve closed system processes. Closed systems minimize occupational exposure through several means, including by preventing fugitive emissions under normal conditions of use (European Chemicals Agency, 2016). However, smaller facilities or other types of operations may have process equipment with a higher potential for emissions.
In the US, the facilities involved in producing and using feedstock for the three chlorinated chemicals are regulated under the Clean Air Act, which requires adhering to Maximum Achievable Control Technology (MACT) standards to minimize evaporative emissions. Moreover, carbon tetrachloride also is tightly regulated under the Montreal Protocol on Substances that Deplete the Ozone Layer. These regulations reduce potential exposures in chemical manufacturing operations and in facilities using these chemicals as feedstocks. For several dermal occupational exposure scenarios within these COUs, however, EPA risk evaluations concluded that potential exists for unreasonable risk of health effects, even in some scenarios in which use of personal protective equipment (PPE) was assumed. Identifying dermal exposures for COUs that have very little or even no skin contact demonstrates the apparent disconnect between actual workplace conditions and estimated occupational dermal exposure in some TSCA risk evaluations. These chemicals were thus selected to evaluate TSCA’s occupational exposure assessment approach.
The objectives of this analysis were to review and evaluate the EPA dermal exposure assessment methodology both generally and as applied in three TSCA chlorinated chemical risk evaluations and to outline best-practice recommendations for regulatory dermal exposure assessments based on the analysis. While the scope of this review and associated case studies was largely limited to scenarios representative of chemical manufacturing and processing as a reactant or intermediate COUs, TSCA also is responsible for evaluating numerous other downstream industrial and consumer users of chemical-containing products. The best practices recommendations are intended to be broadly applicable to occupational dermal exposure assessment for any scenario. However, some of the specific assumptions discussed below and used in modeling would need to be adjusted if applied to downstream users.
Methods
In this analysis, the dermal exposure assessment methodology used in TSCA risk evaluations was evaluated in a two-step process, including: 1) an evaluation of the general exposure assessment framework relative to available occupational health agency and professional association guidance and recommendations; and 2) an evaluation of the EPA’s application of dermal modeling for exposure estimation, including conducting additional occupational exposure modeling. The primary sources consulted for the qualitative methodology review included dermal exposure assessment guidance published by the American Industrial Hygiene Association [AIHA] (American Industrial Hygiene Association, 2015; Stefaniak et al., 2011), the European Chemicals Agency (European Chemicals Agency, 2016), and the European Union’s RISKOFDERM project (e.g., Rajan-Sithamparanadarajah et al., 2004). These sources were supplemented with information obtained from peer-reviewed literature searches on dermal exposure assessment conducted in PubMed. Specifically, several searches were run using a combination of keywords intending to target methodology-based papers (keywords: “exposure assessment methodology” OR “occupational dermal exposure” OR “dermal risk assessment”; and separately (“dermal exposure modeling” OR “dermal exposure model”) AND (“occupational” OR “work” OR “workplace”). Forward searching also was conducted to identify other potentially useful articles (e.g., articles that were listed as “similar to” or “cited by” highly relevant articles in PubMed).
For the second part of the analysis, alternative modeling scenarios were run for non-occluded dermal exposure scenarios for perchloroethylene (PCE), carbon tetrachloride (CTC), and trichloroethylene (TCE) using IH SkinPermTM, a Microsoft Excel-based modeling tool developed by the AIHA Exposure Assessment Strategies Committee (Tibaldi et al., 2014; American Industrial Hygiene Association, 2021; Tibaldi et al., 2017; ten Berge et al., 2020). This model was pre-selected based on previous experience and the understanding that while there is no single consensus modeling method for assessing skin exposure, IH SkinPerm is well accepted in the industrial hygiene community. IH SkinPerm was developed specifically for performing refined dermal risk assessments under various occupational exposure scenarios, such as instantaneous deposition (e.g., a splash), deposition over time (e.g., repeated exposures), and dermal vapor absorption (Tibaldi et al., 2014). Validation testing indicated that in most cases, IH SkinPerm estimates were similar or more health-protective than the empirical data to which they were compared (Tibaldi et al., 2014). IH SkinPerm predicts the dermal flux of a chemical of interest using the physicochemical properties of the chemical, pH of the skin surface, dose estimates, skin surface area, Quantitative Structure-Activity Relationships (QSAR), a differential equation skin transport model, and task duration. This model strikes a balance because it allows for flexibility in inputs based on specific exposure scenarios, but is relatively simple to run.
Finally, based on our review of agency guidance on occupational exposure assessment, published literature, and the IH SkinPerm analyses, we compiled a recommended best-practice framework for TSCA occupational dermal exposure assessments.
Results
Evaluation of the TSCA risk evaluation approach
Overarching methodology
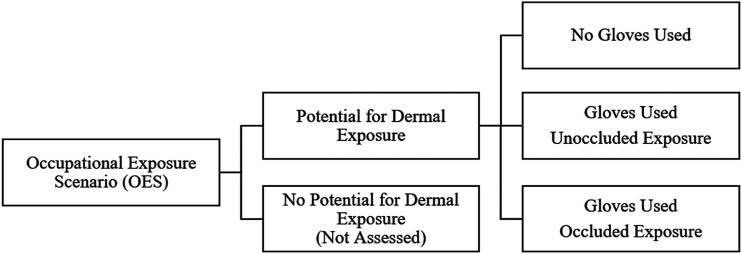
In the TSCA approach to occupational exposure assessment, the evaluation team gathers information on occupational uses from existing records, such as Chemical Data Reporting (CDR) submissions and other public data sources to determine the COUs that EPA will evaluate (Environmental Protection Agency, 2020a). COUs are then divided into subcategories associated with specific occupational exposure scenarios (OES) (e.g., “incorporation into formulation, mixture or reaction product in cleaning and degreasing products” as a subcategory of processing), for the purposes of determining exposure and possible health risks. OESs are intended to capture the specific activities within a COU associated with potential exposure; they also allow for more granular categorization of multiple facility types within a broader COU. EPA calculates the maximum possible dermal exposure concentration for each scenario. Within each OES, dermal exposures were assessed for some or all of three potential exposure scenarios (Figure 1).
Figure 1.
Dermal exposure scenarios evaluated in the first 10 Toxic Substances Control Act risk evaluations.
EPA’s OESs often are not as refined as similar exposure groups (SEGs) in facility-based occupational risk assessments, which are defined based on very specific jobs or tasks in a facility (e.g., operators, maintenance workers, and laboratory technicians). For example, in the first ten risk evaluations, some OESs were as broad as “manufacturing.” Additionally, EPA typically does not conduct qualitative assessment of dermal exposure potential in each OES prior to conducting semi-quantitative exposure estimates (e.g., to exclude specific COUs or subcategories for which dermal exposure is unlikely). EPA’s rationale regarding whether or not dermal exposure potential exists within a given COU subcategory is unclear.
When estimating exposure under TSCA, EPA employs a hierarchical approach in which empirical sampling data are given preference, if available, as the basis for the exposure assessment (Environmental Protection Agency, 2020a). TSCA has a detailed literature search and selection strategy, including processes for identifying unpublished gray literature, to collect exposure information (Environmental Protection Agency, 2021b). In many cases, however, empirical dermal exposure data are not available, and thus modeling is employed.
Many factors introduce uncertainty in dermal modeling, including variation in skin contact frequency and nature, inter- and intra-human absorption variability, and the absence of biological dose area metrics and dose deposition location conventions, among others (Stefaniak et al., 2011). Perhaps in light of these uncertainties, EPA recently issued Section 4 test orders for nine of the next 20 high-priority chemicals to undergo risk evaluations under TSCA (Environmental Protection Agency, 2021c). How EPA intends to use these data in subsequent risk evaluations is currently unclear.
From an overall methodology standpoint, EPA’s hierarchical approach differs from other occupational agency guidelines and international regulatory bodies that often incorporate tiered approaches—notably, AIHA, as described in its “White Book” (Stefaniak et al., 2011) and the European Chemical Agency’s approach under the Registration, Evaluation, Authorisation and Restriction of Chemicals regulation (REACH; see Van de Sandt et al. (2007). The peer-reviewed literature also promotes the use of tiered assessment approaches for occupational exposure assessment (Fantke et al., 2020). Broadly, a tiered assessment approach begins with qualitative data gathering, including facility inspections, surveys, and/or informational interviews to determine whether dermal exposure is possible, and, if so, where in the processes. Groups of workers (SEGs) with similar dermal exposure potential are defined based on specific tasks (e.g., loading/unloading of raw chemical), and the assessor determines whether there is a need for data collection, quantitative exposure and risk assessment, and/or risk management strategies (Jahn et al., 2015). As noted above, there is no indication that EPA’s current approach includes qualitative evaluation of the relative exposure potential within COUs as a first step in the exposure assessment. In the chlorinated chemical assessments, for example, empirical data were not identified, and EPA moved directly to the modeling approach described below.
Risk evaluation models and input parameters
EPA used similar general modeling methods for dermal exposure assessment for the volatile, liquid chemicals, with slight modifications based on the physical state of each chemical, the chemical and industry-specific COU, and physicochemical properties. The Dermal Exposure to Volatile Liquids (DEVL) model was used to estimate occupational dermal exposures for unoccluded scenarios. This model is a modified version of the EPA/OPPT 2-Hand Dermal Exposure to Liquids Model (Environmental Protection Agency, 2020a). For occluded scenarios, a simple mass-balance equation was used assuming no evaporation.
Both the DEVL model and the mass-balance models are steady state, event frequency-based dermal dosage models that estimate the dermal retained dose (see equations (1) and (2); variables are defined in Table 1). As EPA notes, these models do not address exposure duration and frequency variability (Environmental Protection Agency, 2020a, 2020b). The user must select six inputs, detailed in Table 1. Additionally, in the first ten risk evaluations, EPA calculated dermal exposure incorporating a protection factor (PF) in order to account for protection from gloves (see equation (1)). Because some inputs are chemical-specific, we evaluated the TSCA methodology for selected chlorinated chemicals that were among the first ten risk evaluations.
| (1) |
| (2) |
Table 1.
Comparison of Toxic Substances Control Act risk evaluation model default input parameters to contemporary industry conditions for chlorinated chemicals.
| Parameter | Default value or selection | EPA rationale in risk evaluation | Potential limitations in EPA assumptions |
|---|---|---|---|
| S Surface area |
CT: 535 cm2
HE: 1070 cm2 |
Surface area of one or two hands for CT and HE,
respectively; equivalent to assuming dipping one or
both hands into a chemical container. (Environmental Protection Agency, 2011: Table 7–12)a |
S is likely much less than one full hand and two full hands for CTE and HE, respectively, in chemical manufacturing with training and advanced hygiene programs.b Any hand contact unlikely for closed systems.c,d |
| Qu Quantity remaining on skin |
CT:
1.4 mg/cm2/event HE: 2.1 mg/cm2/event |
Based on default values from the EPA/OPPT
2-Hand Dermal Exposure to Chemicals
Model. Constant default parameter that
cannot be changed; represents the quantity that
remains as a film on the skin after the bulk liquid
has fallen off the hand surface. Assumes no hand
washing for 8 h. (Environmental Protection Agency, 2015: Table B-13)e |
For volatile chemicals or those with high rates of absorption, Qu should not be constant.e,f EPA Qu values do not account for variable skin loading intensity as a function of the operation nor do the Qu values allow for duration-adjustable loading values. |
| Fabs Fraction of chemical absorbed |
Non-occluded: Chemical-specific (e.g., 13% for PCE, 8–13% for TCE, 4% for CTC) |
Values derived based on a diffusion model developed by Kasting and Miller 2006.g Fabs is the fraction of applied chemical mass that is absorbed into the skin (the rest evaporatesh). Values are based on numerous factors, including air speed in industrial or commercial settings, octanol:water partition coefficient, molecular weight, water solubility, and vapor pressure. | This approach does not factor in hand movement and skin saturation for a specific scenario. Fabs for a volatile chemical and short exposure duration is expected to be low because of high evaporative flux relative to dermal flux.i |
|
Occluded: 100% |
Occlusion assumes solvent splashes under the cuff of the glove and moves within the glove to coat the hand surface. The default used in the REs is an fabs of 100%, which assumes all solvent is absorbed into the skin over the course of 8 h during which the glove is not removed and replaced. | Assumption is unlikely in chemical production because of glove hygiene standards (change out)j, dilution with sweatk, and flux back out of glove. | |
| BW Body weighth |
80 kg | EPA Exposure Factors Handbookl,m | Typical defaults range between 70 and 80 kg as an average or median.l,m,n |
| Yderm Weight fraction |
1 (100%) | Default value for the chemical concentration if lacking specific data. A Yderm of 1 is the maximum value, in which 100% of the chemical formulation or product is assumed to contain only the chemical of interest.o Alternative weight fractions are used for some OESs. | Yderm should be chemical/formulation- and task-specific; Yderm may be 100% on some occasions, but more than likely occurs within a mixture or formulation of less than 100%, particularly for closed systems in which systems are purged prior to line opening. |
| FT Exposure event frequency |
1 event/day | Assumes that for all dermal scenarios, there was one exposure event (applied dose) per workday with a steady-state fractional absorption rate achieved over 8 h, based on the existing framework of the EPA/OPPT 2-Hand Dermal Exposure to Liquids Model. | May not reflect actual behaviors; typically hands are washed/gloves removed within 1–2 h of tasks and immediately if chemical contact is observed by the wearer.p,q |
Notes: CTC = carbon tetrachloride; CT = central tendency; HE = high end; PCE = perchloroethylene; TCE = trichloroethylene; EPA = Environmental Protection Agency.
bAlliance (2021) (SOPs for Personal Protection at CTC Manufacturing Sites).
hFor very small doses of high volatile substances, the film on the skin would likely evaporate “before a significant amount of permeant has crossed the [stratum corneum]” (Kasting and Miller, 2006).
lEPA provides several versions of the dermal exposure equation across risk evaluations, sometimes including body weight (and results in mg/kg/day) and other times not. To determine whether there is an unreasonable risk, margins of exposure (MOEs) are presented in mg/kg/day (or for cancer risk, per mg/kg-day), and compared against the estimated dermal doses in mg/kg/day.
mEnvironmental Protection Agency (2011), OSHA 29 CFR 1910.141, and 1910.1200. OSHA 29 CFR 1910.141 requires that “employers shall provide handwashing facilities” and 29 CFR 1910.1200 details the Hazard Communication Standard, including standard labels that note to “wash hands thoroughly after handling.”
pImmediate hand washing has high efficacy for chemical removal, even without soap for some substances, as demonstrated, for example, in Forsberg et al. (2020).
First, we analyzed the similarities and differences between the general approach used in the risk evaluations relative to typical chemical industry industrial hygiene approaches for characterizing occupational dermal exposure. The overall results are summarized in Table 1, with associated references in footnotes.
To summarize, the general scenario used in the chlorinated chemical risk evaluations for both occluded and non-occluded scenarios is that a worker’s full hand (for central tendency [CT] estimates), or two full hands (for high-end estimates) comes into contact with neat, undiluted liquid (equivalent to dipping one or both hands into a container of 100% pure chemical). An estimated amount then remains as a film on the skin (Qu; see Table 1) without hand washing for the full workday. A percentage of the amount on the skin is then absorbed, depending on physicochemical properties. The assessment assumes one exposure event per day, with no specific exposure duration; thus, following contact, penetration through the layers of the skin effectively continues unimpeded. The TSCA risk evaluation methodology inherently assumes a worst-case, and possibly unreasonable scenario, in which workers do not remove and replace gloves with visible liquid penetration or wash their hands at any point after exposure.
In reality, in chemical manufacturing, dermal exposure (were it to occur) would only occur intermittently and for discrete period(s) of time (e.g., a 15-min loading task, repeated any number of times throughout a shift). Assuming regular handwashing as required by OSHA (including prompt handwashing in instances of contamination), the majority of chemical residue left on the skin after accounting for evaporation would likely be washed off during the workday (Forsberg et al., 2020).
Notably, if EPA had gathered information from a qualitative observational study of facilities, manufacturing tasks may have been excluded prior to any semi-quantitative exposure estimation, based on the lack of exposure in closed systems and procedural controls that mitigate any skin contact.
Additional considerations for occluded scenarios
For occupational exposure scenarios under which occlusion is expected, the risk evaluation provided estimates of the dermal potential dose rate (mg/day) using a simplistic mass-balance equation (equation (2); Table 1). The calculations represent a scenario in which liquid chemical enters the cuff of the glove and moves within the glove to coat the entire surface of the hand, thereby enhancing dermal penetration. This calculation does not account for the low likelihood that liquid permeating all the way through the glove would coat the entire hand, or that liquid spilled over the cuff would typically result in removal of the glove. Conceptually, this approach approximates the concept of “infinite dose,” as is reflected in in vivo dermal studies and in some in vitro and ex vivo dermal penetration studies (e.g., OECD Test Guideline 428). An actual exposure would likely have lower amounts of chemical entering gloves, a smaller affected hand surface area, sweat in the glove (lowering absorption), and, in many cases, gloves with a higher protection factor, as discussed below (Cherrie et al., 2004).
Glove protection factors
In the first ten draft risk evaluations, where applicable, EPA assessed the impact of protective equipment (gloves) on estimated dermal exposure and risk in the form of protection factors (PFs) for various protection levels, which represent the potential protective effect of glove use. The PFs are applied to the derived internal doses from dermal exposure to modify the overall internal dose. In the risk evaluations reviewed for this analysis, potential health risks for workers handling chemicals without gloves was assessed for one or both of the following scenarios:
1) Using gloves, assuming overall glove PFs of 1, 5, 10, or 20. These scenarios assume there are no occluded exposures (Table 2).
2) Using gloves under occluded conditions (e.g., some chemical penetrates through or splashes under the cuff of gloves and remains trapped, enhancing dermal exposure and penetration).
Table 2.
Summary of dermal protection factors.
| Dermal protection characteristics | Affected user group | Indicated efficiency (%) | Protection factor, PF |
|---|---|---|---|
| a) Any glove /gauntlet without permeation data and without employee training | Both industrial and professional users | 0 | 1 |
| b) Gloves with available permeation data indicating that the material of construction offers good protection for the substance | 80 | 5 | |
| c) Chemically resistant gloves (i.e., as b above) with “basic” employee training | Industrial users only | 90 | 10 |
| d) Chemically resistant gloves in combination with specific activity training (e.g., glove removal and disposal procedures) for tasks in which dermal exposure can be expected to occur | 95 | 20 |
Adapted from: Environmental Protection Agency (2020c: p. 212)
Both scenarios assume that a worker wears the same pair of gloves for the entire work shift (8 h), and that 100 percent of the chemical that enters the glove is absorbed. The latter assumption neglects the potential for reaction with or sequestration in the glove matrix and flux of the chemical mass back out of the glove via transport and evaporation during periods of no liquid contact. Some fraction of the chemical, although assumed small, will also leave the glove through evaporation around the cuff.
The four protection factors applied in the risk evaluations are based on the strategy presented in the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) Targeted Risk Assessment (TRA) model, which was evaluated by Marquart et al. (2017) by comparing the model performance with independent measured dermal exposure levels. Regarding glove efficacy, six of the eleven studies had an average exposure reduction of > 95% for gloves, which would yield a PF > 20. Marquart et al. (2017) concluded that in the model, “the effect of gloves is underestimated if the reasonable worst-case defaults used in regulatory risk assessment practice are used” (p. 868). Similarly, the German Federal Institute for Occupational Safety and Health (FIOSH) evaluated 142 datasets on glove efficacy and found an average PF of 88%, noting that based on distributions, the majority of PFs were higher than 88%. However, FIOSH also noted that given some of the study limitations and the substantial method variability across studies, default PFs could not be “substantiated nor disproven” based on the empirical data evaluated (Oltmanns et al., 2016).
The TSCA PFs are assumed to reflect scenarios in which gloves tear, change out schedules are ineffective or not followed, or other “worst case” personal protective equipment (PPE) use patterns. These situations are possible, particularly in certain small downstream industries. However, these conditions are far less common for tasks with direct chemical exposure at chemical manufacturing facilities. By law, OSHA requires using hand protection to guard against “absorption of harmful substances” (Occupational Safety and Health Administration, 2019). In a facility with an advanced hygiene program, glove selection and usage is consistent with workplace conditions, potential exposure duration, hazard type, and other factors, and includes donning and doffing procedures (Occupational Safety and Health Administration, 1994, 2019). Glove types are selected to ensure suitability for the specific chemical used based on empirical breakthrough test data supplied by the manufacturer or other companies, with an allowable use time and replacement schedule that aims to avoid chemical breakthrough for the specific task duration (Stenzel et al., 2015). Furthermore, any detectable breakthrough or glove degradation would indicate the need for new gloves. Situations in chemical manufacturing with full glove coverage of liquid material are rare, but in such a situation, specific controls (e.g., an inner glove or taped sleeves) identified by a job hazard analysis would be in place to limit dermal contact (Occupational Safety and Health Administration, 2002). Thus, with an advanced industrial hygiene program in chemical manufacturing, dermal exposure is expected to be de minimis, although not always zero.
Results of alternative modeling approach using IH Skin Perm
Given that the TSCA mass-balance model does not allow for consideration of exposure duration, and some of the model inputs may not reflect current industry practices, IH SkinPerm modeling was conducted to evaluate the impact of incorporating duration and alternative exposure inputs that reflect more likely chemical manufacturing OESs. The inputs selected, as applied to PCE, are described below. The same approach was applied to TCE and CTC (see Table 5 and Supplementary Materials for results).
Table 5.
Comparison of total absorbed dose estimates from the risk evaluation and IH SkinPerm.
| Chemical name | EPA Non-occluded acute retained dose (mg/kg-day) |
IH
SkinPerm Non-occluded instantaneous dosea (mg/kg-day) |
Approximate factor-fold differenceb | IH
SkinPerm Non-occluded constant dosea (mg/kg-day) |
Approximate factor-fold differenceb | |
|---|---|---|---|---|---|---|
| Perchloroethylene (PCE)c | CT: | 1.2 | 0.06 | 20 | 0.06 | 20 |
| HE: | 3.5 | |||||
| Trichloroethylene (TCE)d | CT: | 0.8 | 0.20 | 4 | 0.31 | 3 |
| HE: | 2.3 | |||||
| Carbon tetrachloride (CTC)e | CT: | 0.37 | 0.10 | 4 | 0.15 | 2 |
| HE: | 1.1 | |||||
aAssumes 8, 15-min (0.25 hr) events per day.
bCompared to CT value reported by EPA.
cEPA CT and HE estimates are for Bin 1 workers with no glove use (Environmental Protection Agency, 2020a); assumes one applied dose per workday and that 13–19% of the applied dose is absorbed through the skin.
dEPA CT and HE estimates converted from mg/day to mg/kg-day by dividing non-occluded worker dermal retained dose for Bin 1, no glove use, by 80 kg (Environmental Protection Agency, 2020d); assumes one exposure event per day and that 8–13% of the applied dose is absorbed through the skin.
eEPA CT and HE estimates are for workers, all conditions of use, with no glove use (Environmental Protection Agency, 2020a); Estimate assumes one exposure event per day and that 4% of applied dose is absorbed through the skin.
Alternative Analysis: IH SkinPerm and Flux-Based Modeling
As described in Table 3, inputs to the model were modified, as needed, given that IH SkinPerm differs from DEVL in its required inputs. Two scenarios were modeled:
Scenario 1—Instantaneous dermal deposition (e.g., hand wetting or soaking during loading/unloading); and
Scenario 2—Constant deposition over time (e.g., splashing over the course of a task; dripping or minimal contact loading through incidental exposure).
Table 3.
IH Skin Perm parameters for instantaneous and constant dose scenarios and comparison to parameters used by the Environmental Protection Agency.
| Parameter | Instantaneous Dose |
Constant dose | Rationale |
|---|---|---|---|
| Scenario parameters | |||
| Instantaneous deposition dose | 749 mg/event | N/A | Calculated using EPA’s assumed Qu and S values |
| Dermal deposition rate | N/A | 5.6 mg/cm2/hr (assuming 15 min event) | Based on EPA estimation of Qu (quantity remaining on skin) |
| 2.8 mg/cm2/hr (assuming 30 min event) | |||
| 1.4 mg/cm2/hr (assuming a 1 hr event) | |||
| Affected skin area | 535 cm2 | Same as EPA (one-hand) | |
| Maximum skin adherence | 0.648 mg/cm2 | Based on recommendations for IH SkinPerma | |
| Thickness of stagnant air | 1 cm | Default for bare handsa | |
| Weight fraction of PCE | 1 (100% PCE) | Maximum used by EPA (occluded) | |
| Time parameters | |||
| Start of deposition | 0 hr | Assumes deposition begins immediately | |
| Duration of deposition | N/A | 0.25, 0.5, or 1 hr | Same as EPA for 0.5 hr (tank truck) or 1 hr (railcar); additional 0.25 hr added to represent short task (connecting hose) |
| End time observation | 0.25, 0.5, or 1 hr | ||
Notes: PCE = perchloroethylene; EPA = Environmental Protection Agency.
The inputs for IH SkinPerm are summarized in Table 3, and the results are described below.
Notably, in both scenarios, the PCE weight fraction was assumed to be 100%, consistent with the risk evaluation assumption. A formulated product may contain less PCE by weight, estimated to range from 20 to 99% (Environmental Protection Agency, 2020c). Common tasks with PCE that do not involve neat concentrations include handling formulated products, unloading PCE products into mixing vessels, collecting QC samples, packaging formulated products into containers and tank trucks, and handling PCE-containing waste. Dermal exposure estimates that assume maximum weight fraction may therefore be an overestimate of exposure potential in many cases.
Instantaneous dose
For Scenario 1, an instantaneous dermal deposition was modeled, mimicking hand wetting or soaking during loading and unloading tasks. Unlike the DEVL model, IH SkinPerm allows a maximum skin adherence of 10 mg/cm2 for liquids to reflect that excess chemical may not physically be able to stay on the skin. IH SkinPerm also accounts for the fact that air in direct contact with the skin is not fully mixed with the ambient air, essentially forming a stagnant “layer” of air above the skin surface (assumed to be a thickness of 1 cm for bare skin, and 3 cm for light clothing). The instantaneous dose model also allows for a deposition duration that can simulate a task duration, such as rail car or tank truck loading and unloading.
Constant dose
In Scenario 2 (constant dose), the mass PCE loading was uniformly distributed over a 0.25 h, 0.5 h, or 1 h exposure event rather than being instantaneously loaded at the event’s beginning. This scenario more accurately represents splashing over the course of a task, with dripping or minimal contact loading. Based on the parameters for modeling exposure during loading and unloading, the scenario assumed one tank truck per eight-hour work day for the CT scenario, or one railcar averaged over an eight-hour work day (Environmental Protection Agency, 2020a). The inputs are provided in Table 3.
IH SkinPerm results
The IH SkinPerm instantaneous and constant PCE dose estimates are summarized in Table 4. For Scenario 1, instantaneous PCE dermal deposition modeled over a duration of 15 min to 1 h resulted in an absorbed fraction between 0.0813 and 0.325%. This absorption fraction is 40 to 160-fold lower than the 13% absorption fraction used in the risk evaluation, as it accounts for both evaporation and bulk loss. The estimated total dermal dose for 4 to 8 to contact loadings ranged from 0.061 to 0.244 mg/kg/day, which is approximately 5 to 20-fold lower than EPA’s CT estimate of 1.2 mg/day (Table 4). For tasks common to chemical manufacturing, lower absorbed doses are much more likely than those estimated using a continuous loading dose scenario.
Table 4.
Summary of IH SkinPerm results for instantaneous and constant dose scenarios for perchloroethylene.
| Scenario 1 - Instantaneous dermal exposure | |||
|---|---|---|---|
| Observation time | Absorbed fraction % (fabs) | Amount absorbed (mg) per event | Total absorbed dose per workdaya (mg/day) (mg/kg/day) |
| 15 min (0.25 hr) | 0.0813% (0.000813) | 0.609 | 4.87 (0.0609) |
| 30 min (0.5 hr) | 0.163% (0.00163) | 1.22 | 4.88–9.76 (0.061–0.122) |
| 60 min (1 hr) | 0.325% (0.00325) | 2.44 | 19.52 (0.244) |
aFor 15 min duration—assume application of 8 times per day; for 30 min duration—assume application 4 or 8 times per day; for 60 min duration—assume 8 events per day.
The results for Scenario 2 demonstrate that a constant PCE dose uniformly loaded on the skin (15-min to 1-h duration) results in 0.0796%–0.318% absorbed. This absorption fraction is approximately 41 to 170-fold lower than the 13% fraction that the risk evaluation assumes. The estimated total dermal absorbed doses for 4 to 8 contact times per day ranged from 0.0596 to 0.238 mg/kg/day, approximately 5 to 20-fold lower than EPA’s CT estimate of 1.2 mg/kg/day (Table 4). Scenarios of frequent, constant dose loading are relatively rare in a well-managed chemical manufacturing environment, as they represent nearly continuous contact with liquid PCE over the course of an 8-h workday. The model assumes, however, that absorption in the skin stops at the end of the observation time, whereas absorption may continue past observation time for the residual chemical volume that is not completely washed from the stratum corneum. Thus, in the unlikely event that this exposure pattern were to occur in a chemical manufacturing environment, the model estimate presented could underestimate maximum uptake potential, depending on the chemical’s ability to diffuse out to the skin layers once exposure ended.
The dermal exposure assessment approach used in the TSCA PCE risk evaluation includes various default, scenario-centric parameters that are applied without considering exposure duration. When IH SkinPerm was used, and exposure duration and skin saturation were considered, the estimated dermal doses were substantially lower than the risk evaluation estimates. The dermal approach used in the PCE risk evaluation may have overestimated:
• The PCE absorption fraction by 20 to 160-fold for exposure to an ungloved hand.
• The total PCE dermal dose by approximately 5 to 20-fold for an ungloved hand, depending on the number of exposure events per day.
These modeling scenarios reflect well-characterized industrial handling practices, while still yielding health-protective estimates.
Application to other chlorinated chemicals
Similar analyses were performed for TCE and CTC. The IH SkinPerm output is detailed in the Supplementary Materials (Tables S.1-S.4). A comparison between the approach used in the risk evaluation and the IH SkinPerm model for estimated total absorbed dose from non-occluded dermal exposure to three chlorinated chemicals is summarized in Table 5.
For all three chemicals, the non-occluded absorbed doses are likely overestimated by 2 to 20-fold, depending on the chemical and modeled exposure scenario.
Discussion and recommendations
Our preliminary analysis demonstrated that the modeling approach used for chlorinated chemicals in TSCA risk evaluations may have overestimated total dermal absorbed doses by 2- to 20-fold for relevant exposure conditions in chemical manufacturing. Based on our findings, several recommendations are suggested for improving the occupational dermal exposure assessments in TSCA risk evaluations.
As discussed in the results section, the current occupational exposure assessment approach under TSCA does not appear to follow a tiered structure. TSCA dermal occupational exposure assessments should begin with qualitative data gathering, including facility inspections, surveys, and/or informational interviews to determine whether dermal exposure is possible, and, if so, at what point in the processes. If dermal exposure potential is identified, EPA may choose to perform lower-tier modeling, but this modeling should use information collected in the data gathering step. If unreasonable risks are determined based on these lower- or mid-tier models that overestimate exposure, additional higher-tier models or additional analysis should be required prior to risk management.
Qualitative information gathering and surveys
Qualitative information gathering is exposure assessment’s first tier and can help prioritize scenarios and improve confidence in risk assessments (Stefaniak et al., 2011). Qualitative information can be collected by field observations or by soliciting surveys that better characterize the current tasks at manufacturing facilities, including information on task duration, contact volumes and frequencies, and PPE practices for incorporating into the modeling assumptions.
Qualitative field observations and data gathering are recommended to determine where dermal exposure potential is possible, and to rule out processes and tasks with no dermal exposure potential. There is little (if any) industrial chemical dermal contact during routine tasks at facilities (ECHA, 2016) with closed system process units, such as the case with producing the three chlorinated chemicals discussed as case examples, as well as their use as feedstocks for other chemicals. Scenarios with closed systems can likely be excluded from more complex analysis during the qualitative workflow step.
After identifying tasks with some level of dermal exposure potential, semi-quantitative screening-level approaches are recommended prior to moving to higher-tier validated empirical sampling and/or modeling for developing quantitative estimates of exposure.
Semi-quantitative approaches
In cases where the qualitative assessment indicates the need for a semi-quantitative or quantitative analysis, dermal exposure models varying from screening level to refined can be used to identify potential exceedances of a risk-based benchmark (Stefaniak et al., 2011; Van de Sandt et al., 2007). Modeling provides a numeric value for the dermal dose, but because models approximate exposure and are not direct quantitative dermal exposure measurements (which would need empirical sampling), they are considered “semi-quantitative.” Screening-level models are intentionally designed to overestimate exposure. As the assessor progresses through modeling tiers, the amount of data required for exposure estimates increases, but so too does the level of accuracy in the estimates. When using a tiered approach, an industrial hygienist collects data to refine the exposure estimate until better able to estimate actual exposure conditions.
The DEVL model, for example, is considered a low-tier model and would thus be expected to generate overestimates of exposure based on the inputs used in the chlorinated chemical risk evaluations (e.g., surface area; task duration; and weight fraction). Steps as simple as refining these inputs and employing tools like IH SkinPerm would be considered a more refined, higher-tier semi-quantitative approach for determining if a potential risk remains, given the inputs based on a deeper understanding of industry conditions. If these more refined estimates continue to show potential risks for some tasks within an OES, then these tasks could be prioritized for additional higher-tier methods and risk management (i.e., implementation of further controls).
Because of the substantial lack of empirical data on skin absorption and dermal doses for many chemicals, when qualitative observation indicates dermal exposure potential, modeling estimates may be needed. In such cases, best practices should include using a well-accepted, peer-reviewed modeling approach (e.g., IH SkinPerm). Furthermore, a well-validated model will only produce reasonable estimates if the inputs are carefully researched. As such, best practices also dictate that modeling be based on assumptions representative of industry conditions (Stefaniak et al., 2011; Tibaldi et al., 2014). Collating current and accurate information regarding the specific characteristics of all tasks with the potential for dermal exposure, and periodically updating the information when industry conditions change, is critical. This process is the essence of job hazard analyses common in chemical handling industries. EPA may consider soliciting surveys from impacted producers/users regarding general uses of the chemical of interest, and detailed information on corresponding task descriptions, duration, contact volumes, and contact frequency.
Empirical studies
For most scenarios, semi-quantitative analyses are sufficient and appropriate for characterizing dermal exposures with well-defined SEGs. Where qualitative information gathering and semi-quantitative exposure measures indicate a high potential for exposure, quantitative exposure estimation using sampling can be employed with an aim to collect sufficient information to select an appropriate control level (Stefaniak et al., 2011).
Importantly, field measurements collected on the skin have demonstrated spatial (e.g., body segment), temporal (e.g., shift or day to day), personal (e.g., work habits or physiology), and sampling (e.g., direct or indirect measures) variability that can often confound designing and executing measurement studies. Limitations of such studies for volatile chemicals have been described in reviews (Cherrie et al., 2004). Further, some of the sampling considerations may not be intuitive, especially for volatile chemicals. Thus, developing such sampling approaches requires a significant method evaluation effort. For volatile chemicals, there are substantial methodological challenges to conducting empirical dermal sampling because of their high vapor pressure.
Several studies have presented general practices for collecting field samples to assess volatile chemical dermal exposure using charcoal cloth pads and solvent indicator sensors, such as Permea-Tec solvent sensors (Creta et al., 2017, 2021; Cohen and Popendorf, 1989; Phanprasit et al., 2019; Vermeulen et al., 2006; Van Wendel De Joode et al., 2005). Developing a dermal sampling methodology that utilizes an adsorbent pad or sensor should be validated in the field, however, to test the methodology feasibility in an uncontrolled industrial or commercial environment. Additionally, while dermal wipes may be useful for solids and non-volatile liquids, collecting dermal wipe samples to characterize VOC exposures over a full shift is unlikely to detect a volatile chemical because of evaporation from the skin before wiping. Overall, empirical dermal sampling is sometimes needed and can be particularly useful in conjunction with models, but for volatile substances, laboratory and field methodology evaluations should be conducted prior to field sampling.
As described above, EPA recently issued Section 4 test orders seeking occupational data for nine of the next 20 high-priority chemicals to undergo risk evaluations under TSCA (Environmental Protection Agency, 2021c). For some chemicals, TSCA Section 4 test orders also include requests for dermal absorption data generated using non-animal methods, such as OECD test guideline 428, an in vitro study using human skin (Organisation for Economic Co-operation and Development, 2004). When using simpler models, such as DEVL, the user must calculate and input a value for fraction absorbed. While a simple diffusion model can be used to estimate absorption, dermal absorption can be more accurately quantified by various in vivo and in vitro methods, such as OECD 428, to refine the occupational dermal assessment. Note, however, that IH SkinPerm estimates dermal absorption based on chemical-specific properties and the task duration and frequency input assumptions, either as an instantaneous absorption or over time (continuous absorption), and thus empirical absorption data are not required. Empirical dermal sampling is sometimes needed, however, and can be particularly useful in conjunction with models, but for volatile substances, laboratory and field methodology evaluations should be conducted prior to field sampling.
PPE assessment
Occupational controls, including PPE, are an important consideration in risk evaluation and risk management. While PPE use is not considered in some screening-level assessments, PPE efficacy and prevalence must be considered at some point in the exposure assessment process since appropriate risk management decisions rely on understanding the hierarchy of occupational controls (Stenzel et al., 2015). Estimating potential exposure while using dermal PPE requires inputs that are reflective of current IH practices, including glove change out schedules, efficacy of gloves worn during specific tasks, and handwashing procedures. As AIHA details and as discussed above, glove use information should be gathered through surveys and other qualitative data gathering (Stefaniak et al., 2011). Glove usage patterns should be assessed for each specific SEG, allowing consideration of dermal exposure potential for each SEG based on the task(s) and whether or not gloves are routinely used.
When assessing glove efficacy and breakthrough, permeability data may be available, or can be gathered using the standard method for glove permeability testing (American Society for Testing and Materials, 2020). Further, all glove manufacturers publish data regarding glove material degradation and breakthrough times. Freely available tools also exist for assessing glove permeability and selecting appropriate PPE, such as the ProtecPo web-based tool (Drolet et al., 2018). When a refined assessment is needed, empirically based protection factors can be derived using experimental data on chemical permeation through gloves, considering critical factors such as chemical content extent and length, amount of hand/glove flexion, and worker behavior (Chao et al., 2004; Cherrie, 2018). Overall, while observational and semi-quantitative estimation of glove efficacy are available, additional empirical validation of these results would increase confidence in the assumption of de minimis exposure.
Summary of recommendations
Overall, we recommend that the TSCA risk evaluation program consider a tiered approach for occupational dermal exposure assessment in its risk evaluations. That approach should prioritize early qualitative information gathering, incorporate careful field data and assumption selection for task frequency, duration, and other parameters, consider commonly used practices for glove selection and change out, and utilize a validated and peer-reviewed model (such as IH SkinPerm) when a modeling estimate is required. A generic, task-based exposure assessment tool, such as RISKOFDERM (used in the EU under REACH), could also be considered in order to increase the cost-effectiveness, accuracy, and simplicity of a tiered dermal assessment approach (Van de Sandt et al., 2007).
Conclusions
When conducting occupational exposure assessment for any route, tiered approaches should be employed to efficiently and effectively evaluate exposure potential. When modeling is used, modeling inputs must be carefully researched and selected to reflect industry practices and conditions as accurately as possible. In the dermal modeling conducted for the first ten high-priority chemicals evaluated under the amended TSCA, numerous differences exist between assumptions used and what is known (and what can be known) about tasks and exposure potential under typical industrial hygiene practices in chemical manufacturing and processing facilities. The high-end exposure scenarios used in the chlorinated chemical risk evaluations are unlikely to occur during tasks with known dermal exposures in chemical manufacturing facilities, and more representative assumptions would result in substantially lower dermal dose estimates. The methods presented in this manuscript provide a recommended framework for best practices for dermal exposure assessment under TSCA- specifically, a tiered approach to occupational dermal exposure assessment that incorporates well-validated models and empirical data when necessary. EPA’s recent TSCA Section 4 test orders to solicit dermal absorption and exposure data represent a crucial step forward for recognizing the importance of robust occupational dermal exposure assessment. The information and data collected and incorporated into a tiered approach beginning with qualitative information gathering can substantially improve occupational exposure estimate accuracy in TSCA risk evaluations.
Supplemental Material
Supplemental material for Analysis of dermal exposure assessment in the US environmental protection agency toxic substances control act risk evaluations of chemical manufacturing by Heather Lynch, Lauren Gloekler, Laura Hallett Allen, Joshua R Maskrey, Christopher Bevan, and Andrew Maier in Toxicology and Industrial Health
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All of the authors are or were employed by Stantec ChemRisk, a consulting firm that provides scientific advice to the government, corporations, law firms, and various scientific/professional organizations, or the Halogenated Solvents Industry Alliance, Inc. (HSIA), a trade organization representing halogenated solvent producers and users. HSIA and the Foundation for Chemistry Research and Initiatives provided funding for preparing this manuscript. The content and conclusions of the manuscript, however, are exclusively those of the authors.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the American Chemistry Council, Halogenated Solvents Industry Alliance, Inc.
Supplemental material: Supplemental material for this article is available online.
ORCID iD
Heather N Lynch https://orcid.org/0000-0002-6140-3482
References
- American Industrial Hygiene Association (2015) A Strategy for Assessing and Managing Occupational Exposures. 4th ed. Falls Church, VA: American Industrial Hygiene Association. [Google Scholar]
- American Industrial Hygiene Association (2021) IH Apps & Tools. Falls Church, VA: American Industrial Hygiene Association. Retrieved July 22, 2021 from: https://www.aiha.org/public-resources/consumer-resources/topics-of-interest/ih-apps-tools [Google Scholar]
- American Society for Testing and Materials (2020) ASTM F739-20, Standard Test Method for Permeation of Liquids and Gases Through Protective Clothing Materials Under Conditions of Continuous Contact. West Conshohocken, PA: American Society for Testing and Materials. www.astm.org [Google Scholar]
- Anderson SE, Meade BJ. (2014) Potential health effects associated with dermal exposure to occupational chemicals. Environmental Health Insights Journal 8(Suppl 1): 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Ahlers HW. (2003) Federal government regulation of occupational skin exposure in the USA. International Archives of Occupational and Environmental Health Journal 76(5): 387–399. [DOI] [PubMed] [Google Scholar]
- Chao K-P, Wang V-S, Lee P-H. (2004) Modeling Organic Solvents Permeation Through Protective Gloves. Journal of Occupational and Environmental Hygiene 1(2): 57–61. [DOI] [PubMed] [Google Scholar]
- Cherrie JW. (2018) How to quantitatively assess dermal exposure to volatile organic compounds. Annals of Work Exposures and Health Journal 62(2): 253–254. [DOI] [PubMed] [Google Scholar]
- Cherrie JW, Semple S, Brouwer D. (2004) Gloves and dermal exposure to chemicals: proposals for evaluating workplace effectiveness. Annals of Occupational Hygiene Journal 48(7): 607–615. [DOI] [PubMed] [Google Scholar]
- Cohen BS, Popendorf W. (1989) A method for monitoring dermal exposure to volatile chemicals. American Industrial Hygiene Association Journal 50(4): 216–223. [DOI] [PubMed] [Google Scholar]
- Creta M, Poels K, Thoelen L, et al. (2017) A method to quantitatively assess dermal exposure to volatile organic compounds. Annals of Work Exposures and Health Journal 61(8): 975–985. [DOI] [PubMed] [Google Scholar]
- Creta M, Savory LD, Duca RC, et al. (2021) An alternative method to assess permeation through disposable gloves. Journal of Hazardous Materials 411: 125045. [DOI] [PubMed] [Google Scholar]
- Dotson GS, Chen CP, Gadagbui B, et al. (2011) The evolution of skin notations for occupational risk assessment: a new NIOSH strategy. Regul Toxicol Pharmacol 61(1): 53–62. [DOI] [PubMed] [Google Scholar]
- Drexler H. (2003) Skin protection and percutaneous absorption of chemical hazards. International Archives of Occupational and Environmental Health Journal 76(5): 359–361. [DOI] [PubMed] [Google Scholar]
- Drolet D, Lara J, Zimmermann F, et al. (2018) ProtecPO: web-based tool using the hansen solubility parameters for the selection of protective polymer materials against chemicals. Presentation at the Australian Institute of.Occupational Hygienists (AIOH) 36th Annual Conference and Exhibition, Melbourne, Motreal, Quebec: IRST-INRS [Google Scholar]
- Environmental Protection Agency (2004) In vitro dermal absorption rate testing of certain chemicals of interest to the occupational safety and health administration: final rule. 69 FR 22402. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (2011) Ch. 7: dermal exposure factors. In: Exposure Factors Handbook. Washington, D.C.: U.S. Environmental Protection Agency. [Google Scholar]
- Environmental Protection Agency (2015) ChemSTEER User Guide: Chemical Screening Tool for Exposures and Environmental Releases. Washington, D.C.: U.S. Environmental Protection Agency. [Google Scholar]
- Environmental Protection Agency (2020. a) Final Risk Evaluation for Carbon Tetrachloride: Supplemental Information on Releases and Occuptational Exposure Assessment. CASRN: 56-23-5. Washington, D.C.: U.S. Environmental Protection Agency-Office of Chemical Safety and Pollution Prevention. [Google Scholar]
- Environmental Protection Agency (2020. b). Final Risk Evaluation for Perchloroethylene - Systematic Review Supplemental File: Releases and Occupational Exposure Assessment. December 2020. Available from: https://www.epa.gov/sites/default/files/2020-12/documents/16_pce_supplemental_information_file_releases_and_occupational_exposure_assessment.pdf (Accessed 01 November 2022). [Google Scholar]
- Environmental Protection Agency (2020. c) Risk Evaluation for Perchloroethylene (Ethene, 1,1,2,2-Tetrachloro-) CASRN: 127-18-4. Document # 740-R1-8011. Washington, D.C.: U.S. Environmental Protection Agency (EPA). Available from: https://www.epa.gov/sites/default/files/2020-12/documents/1_risk_evaluation_for_perchloroethylene_pce_casrn_127-18-4_0.pdf (Accessed 01 November 2022). [Google Scholar]
- Environmental Protection Agency (2020. d) Risk Evaluation for Trichloroethylene. CASRN: 791-0-6. EPA. Document # 740-R1-8008. Washington, D.C.: U.S. Environmental Protection Agency (EPA). Available from: https://www.epa.gov/sites/default/files/2020-11/documents/1._risk_evaluation_for_trichloroethylene_tce_casrn_79-01-6.pdf (Accessed 01 November 2022). [Google Scholar]
- Environmental Protection Agency (2020. e) Risk Evaluation for Carbon Tetrachloride (Methane, Tetrachloro-) CASRN: 56-23-5. Document # 740-R1-8011. Washington, D.C.: U.S. Environmental Protection Agency (EPA). Available from: https://www.epa.gov/sites/default/files/2020-10/documents/1_ccl4_risk_evaluation_for_carbon_tetrachloride.pdf (Accessed 01 November 2022). [Google Scholar]
- Environmental Protection Agency (2021. a) Assessing and Managing Chemicals Under TSCA: Risk Evaluations for Existing Chemicals Under TSCA. Washington, D.C.: U.S. Environmental Protection Agency. Last Updated March 30, 2021. Retrieved July 25, 2021 from: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-evaluations-existing-chemicals-under-tsca [Google Scholar]
- Environmental Protection Agency (2021. b) Draft Systematic Review Protocol Supporting TSCA Risk Evaluations for Chemical Substances Version 1.0: A Generic TSCA Systematic Review Protocol with Chemical-specific Methodologies. Washington, DC: U.S. Environmental Protection Agency (EPA)-Office of Chemical Safety and Pollution Prevention. EPA Doc. #EPA-D-20-031. [Google Scholar]
- Environmental Protection Agency (2021. c) Chemicals Under the Toxic Substances Control Act (TSCA): EPA Issues Test Orders for Nine Chemicals Undergoing Risk Evaluation Under TSCA. Washington, D.C.: U.S. Environmental Protection Agency. Jan. 15, 2021. Last Updated June 10, 2021. Retrieved July 25, 2021 from: https://www.epa.gov/chemicals-under-tsca/epa-issues-test-orders-nine-chemicals-undergoing-risk-evaluation-under-tsca [Google Scholar]
- European Chemicals Agency (2016) Guidance on Information Requirements and Chemical Safety Assessment Chapter R.14-Occupaitonal Exposure Assessment. Version 3.0. No.: ECHA-16-G-09-EN. Aug., 2016. Helsinki, Finland: European Chemicals Agency. [Google Scholar]
- Fantke P, von Goetz N, Schluter U, et al. (2020) Building a European exposure science strategy. Journal of Exposure Science & Environmental Epidemiology 30(6): 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg E, Oberg L, Artursson E, et al. (2020) Decontamination efficacy of soapy water and water washing following exposure of toxic chemicals on human skin. Journal of Toxicology: Cutaneous and Ocular Toxicology 39(2): 134–142. [DOI] [PubMed] [Google Scholar]
- Geer LA, Curbow BA, Anna DH, et al. (2006) Development of a questionnaire to assess worker knowledge, attitudes and perceptions underlying dermal exposure. Scandinavian Journal of Work, Environment & Health 32(3): 209–218. [DOI] [PubMed] [Google Scholar]
- Goede HA, Tijssen SC, Schipper HJ, et al. (2003) Classification of dermal exposure modifiers and assignment of values for a risk assessment toolkit. Annals of Occupational Hygiene Journal 47(8): 609–618. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Berlin A, Gilbert M, et al. (1988) Preventing percutaneous absorption of industrial chemicals: the "skin" denotation. American Journal of Internal Medicine 14(1): 97–107. [DOI] [PubMed] [Google Scholar]
- Halogenated Solvents Industry Alliance, Inc . (2021) SOPs for Personal Protection at CTC Manufacturing Sites. Halogenated Solvents Industry Alliance, Inc. Submitted to US EPA Docket EPA-HQ-OPPT-2020-0592. Available online at: https://www.regulations.gov/document/EPA-HQ-OPPT-2020-0592-0003 [Google Scholar]
- Jahn SD, Bullock WH, Ignacio JS. (2015) A Strategy for Assessing and Managing Occupational Exposures. 4th ed. Falls Church, VA: American Industrial Hygiene Association. [Google Scholar]
- Kasting GB, Miller MA. (2006) Kinetics of finite dose absorption through skin 2: Volatile compounds. Journal of Pharmaceutical Sciences 95(2): 268–280. [DOI] [PubMed] [Google Scholar]
- Lampel HP, Powell HB. (2019) Occupational and hand dermatitis: a practical approach. Clinical Reviews in Allergy and Immunology Journal 56(1): 60–71. [DOI] [PubMed] [Google Scholar]
- Lushniak BD. (2004) Occupational contact dermatitis. Dermatology and Therapy Journal 17(3): 272–277. [DOI] [PubMed] [Google Scholar]
- Marquart H, Franken R, Goede H, et al. (2017) Validation of the dermal exposure model in ECETOC TRA. Annals of Work Exposures and Health Journal 61(7): 854–871. [DOI] [PubMed] [Google Scholar]
- Marquart H, Heussen H, Le Feber M, et al. (2008) Stoffenmanager', a web-based control banding tool using an exposure process model. Annals of Occupational Hygiene Journal 52(6): 429–441. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (2007) NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No.: 2005-149. 3rd Printing. Sept., 2007. Cincinnati, OH: Department of Health and Human Services-Centers for Disease Control and Prevention-National Institute for Occupational Safety and Health. [Google Scholar]
- National Institute for Occupational Safety and Health (2019). In: Daniels RD, Gilbert SJ, Kuppusamy SP, et al. (eds), Current Intelligence Bulletin 69: NIOSH Practices in Occupational Risk Assessment. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health. DHHS (NIOSH) Publication. No. 2020-106; DOI: 10.26616/NIOSHPUB2020106. [DOI] [Google Scholar]
- Occupational Safety and Health Administration (1994) Occupational Safety and Health Standards, 1910 Subpart 9 Personal Protective Equipment, Hand Protection (1910.138). [59 FR 16362, April 6, 1994]. Washington, D.C.: U.S. Department of Labor-Occupational Safety and Health Administration. [Google Scholar]
- Occupational Safety and Health Administration (2002) Job Hazard Analysis. OSHA 3071-Revised. Washington, D.C.: U.S. Department of Labor-Occupational Safety and Health Administration. [Google Scholar]
- Occupational Safety and Health Administration (2019) Occupational Safety and Health Standards, 1910 Subpart H, Hazardous Materials. Hazardous Waste Operations and Emergency Response. 84 FR 21598. Washington, D.C.: U.S. Department of Labor-Occupational Safety and Health Administration. [Google Scholar]
- Oltmanns J, Kaiser E, Schneider K, et al. (2016) Effectiveness of Personal Protective Equipment of Dermal Exposure - A Comparative Survey. Dortmund, Germany: Federal Institute for Occupational Safety and Health. [Google Scholar]
- Organisation for Economic Co-operation and Development (2004) OECD Guideline for the Testing of Chemicals - Skin Absorption in vitro Method. 428. Paris: Organisation for Economic Co-operation and Development. [Google Scholar]
- Phanprasit W, Songpek K, Boonyayothin V, et al. (2019) Inhalation and dermal exposure to toluene among printing workers in a plastic bag factory. Journal of Health Research 33(1): 68–79. [Google Scholar]
- Rajan-Sithamparanadarajah R, Roff M, Delgado P, et al. (2004) Patterns of dermal exposure to hazardous substances in European union workplaces. Annals of Occupational Hygiene Journal 48(3): 285–297. [DOI] [PubMed] [Google Scholar]
- Stefaniak AB, Day GA, Virji MA, et al. (2011) Ch. 20: The skin and the work environment. In: Anna DH. (ed) The Occupational Environment: Its Evaluation, Control, and Management. 3rd ed. Falls Church, VA: American Industrial Hygiene Assocation, pp. 536–559. [Google Scholar]
- Stenzel M, Mulhausen J, Damiano J. (2015) Ch.23: Health Hazard Control. In: Jahn SD, Bullock WH, Ignacio JS. (eds) A Strategy for Assessing and Managing Occupational Exposures. 4th ed. Falls Church, VA: American Industrial Hygiene Association. [Google Scholar]
- ten Berge W, Tibaldi R, Drolet D. (2020) IH SkinPerm MS Excel Workbook (Version 2.3). Software available from AIHA.org. [Google Scholar]
- Tibaldi R, ten Berge W, Drolet D. (2014) Dermal absorption of chemicals: Estimation by IH SkinPerm. Journal of Occupational and Environmental Hygiene 11(1): 19–31. [DOI] [PubMed] [Google Scholar]
- Tibaldi R, ten Berge W, Drolet D. (2017) IH SkinPerm v2.0 - Reference Manual. 26. [Google Scholar]
- Van de Sandt JJ, Dellarco M, Van Hemmen JJ. (2007) From dermal exposure to internal dose. Journal of Exposure Science & Environmental Epidemiology 17(Suppl 1): S38–S47. [DOI] [PubMed] [Google Scholar]
- van Wendel de Joode B, Tielemans E, Vermeulen R, et al. (2005) Dermal exposure assessment to benzene and toluene using charcoal cloth pads. Journal of Exposure Science and Environmental Epidemiology 15(1): 47–50. [DOI] [PubMed] [Google Scholar]
- Vermeulen R, Lan Q, Li G, et al. (2006) Assessment of dermal exposure to benzene and toluene in shoe manufacturing by activated carbon cloth patches. Journal of Environmental Monitoring 8: 1143–1148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Analysis of dermal exposure assessment in the US environmental protection agency toxic substances control act risk evaluations of chemical manufacturing by Heather Lynch, Lauren Gloekler, Laura Hallett Allen, Joshua R Maskrey, Christopher Bevan, and Andrew Maier in Toxicology and Industrial Health