Figure 1.
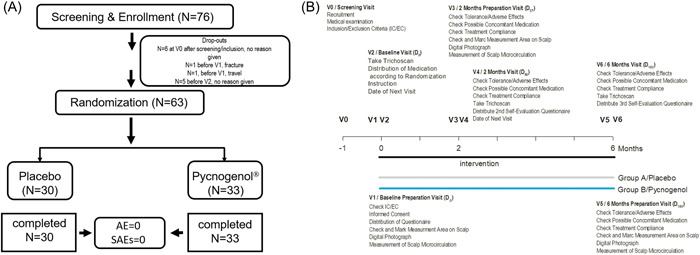
(A) An overall trial design showing number of subjects who finished the study and number of subjects who withdrew including reasons for withdrawal. (B) Details for both groups of the randomized, placebo‐controlled, double blinded study testing the efficacy of the oral supplement Pycnogenol® versus a placebo. At visit 0 (V0) demographic data were obtained, and a medical examination was done. At V1, informed consent was obtained, test area was marked, digital photograph was taken, microcirculation was measured, hair was colored in the test area. At V2, digital photographs were taken, subjects were allocated to either of the two groups A/placebo or B/Pycnogenol,® received medication and were assessed for skin physiological parameters as indicated.
