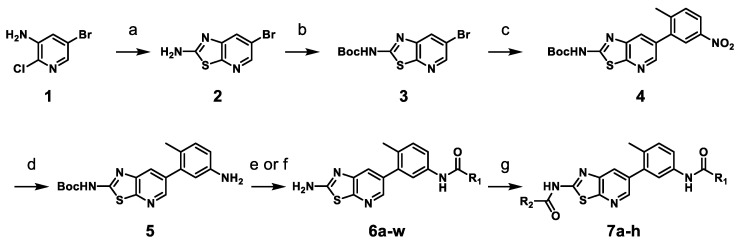
Scheme 1.
Synthesis of thiazolo[5,4-b]pyridine derivatives 6a–w and 7a–ha. a Reagent and condition: (a) potassium thiocyanate, HCl, 100 °C, 12 h, 75%; (b) di-tert-butyl dicarbonate, DMAP, THF, rt, 90%; (c) 2-methyl-5-nitrophenylboronic acid pinacol ester, Pd(dppf)Cl2, Na2CO3, DME/H2O, 100 °C, 3 h, 70%; (d) iron powder, NH4Cl, THF/MeOH/H2O, 70 °C, 1 h, 80%; (e) (i) R1-COOH, HATU, DIPEA, DMF, rt, 6 h; (ii) TFA, CH2Cl2, rt, 6 h, 21–65% (over 2 steps); (f) (i) 3-(trifluoromethyl)phenyl isocyanate, TEA, THF, rt; (ii) TFA, CH2Cl2, rt, 6 h, 53% (over 2 steps); (g) R2-COCl, pyridine, CH2Cl2, rt, 3 h, 21–38%.