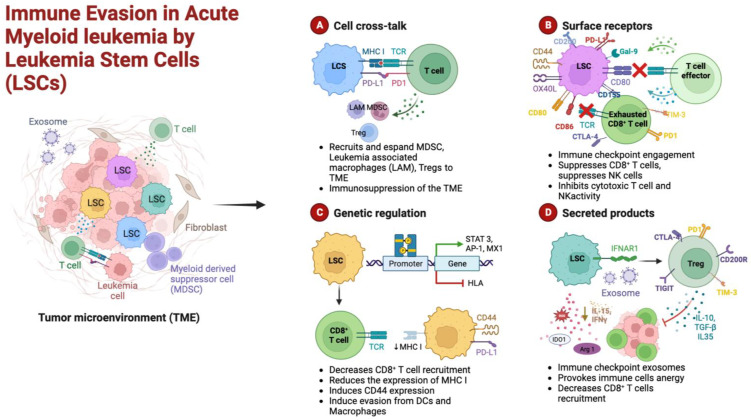
Figure 1.
Schematic representation of the cellular mechanisms of anti-tumor surveillance escape in AML. (A) Cellular cross-talks: leukemic stem cells (LSCs) and T cells direct interaction, through major histocompatibility complex (MHC) and over-expressed immune checkpoint ligands (PD-L1), causes functional defects in anti-tumor immunity. LSCs induce the expansion of immunosuppressive cells such as regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and leukemia-associated macrophages (LAMs). (B) Surface receptors: LSCs can hamper both T- and NK-cell effector functions by aberrantly overexpressing inhibitory ligands such as CD155, CD80, PD-L1, and galectin-9 (Gal-9). (C) Genetic regulation: the activation of immune checkpoint pathways, such as PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and the expression of CD44, a cell-adhesion molecule that has been shown to be involved in the promotion of resistance to drug-induced apoptosis, prevent the appropriate anti-tumor immunity. Moreover, epigenetic mechanisms may result in the downregulation of MHC expression in AML cells, leading to immune escape and relapse in AML. (D) Secreted products: LSCs release inhibitory mediators, either as soluble molecules or as a component of exosomes; furthermore, induced immune cell (Tregs, CD8+ T cells) anergy lowers the levels of inhibitory cytokines. Among these factors that alter the bone marrow microenvironment milieu, a relevant role is played by indoleamine 2,3-dioxygenase (IDO), interferon-gamma (IFN-γ), transforming growth factor beta (TGF-β), arginase 1 (Arg 1), and interleukin 10 and 15 (IL-10 and IL-15). Figure created in Biorender.com.