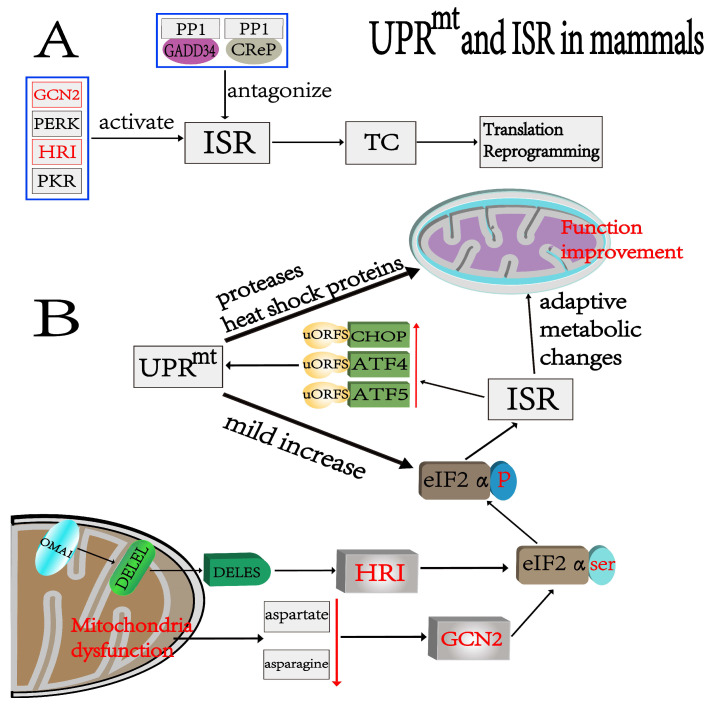
Figure 2.
Relationship between UPRmt and ISR and activation process in mammals. (A) Four kinases, GCN2, PERK, HRI, and PKR, regulate ISR activation, and two other specialized phosphatases antagonize the ISR. The ISR controls protein translation mainly through the TC. (B) Mitochondrial dysfunction induces intracellular stress response mainly through GCN2 and HRI. First, activation of the Oma1 endosomal protein results in conversion of DELEL into DELES. Then, the phosphorylation of eIF-2α serine residues by HRI leads to a series of adaptive metabolic changes that promote the recovery of mitochondrial function and increase the expressions of key UPRmt regulators such as ATF4, ATF5, and CHOP. Second, mitochondrial dysfunction reduces cytoplasmic aspartate and asparagine, which promotes GCN2-induced phosphorylation and, thus, causes corresponding metabolic changes. The activated UPRmt plays a role in maintaining protein homeostasis in mitochondria to enhance mitochondrial function. The UPRmt also causes a mild increase in phosphorylation levels.