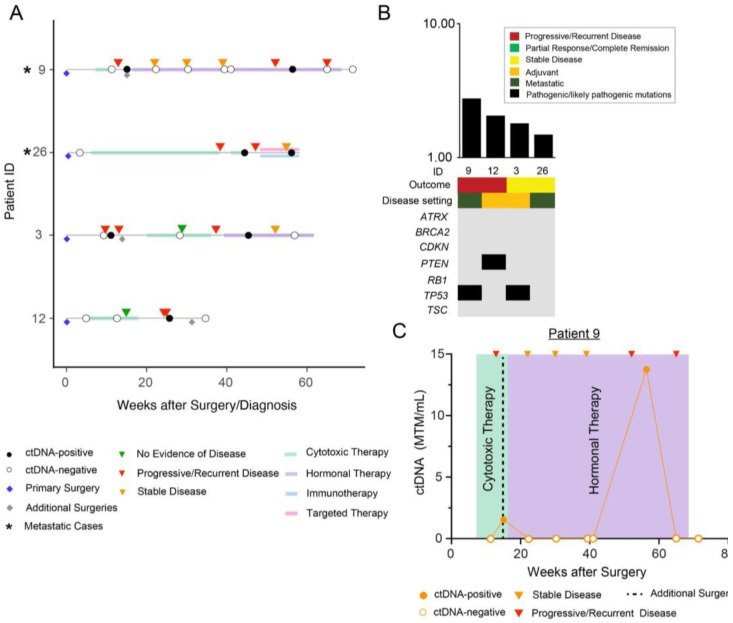
Figure 2.
(A) Overview plot depicting the complete clinical course of all patients within sub-cohort B (initially ctDNA-negative but later became positive), including results of longitudinal ctDNA analysis. Patients with metastatic disease are marked with an asterisk “*” symbol. Blue diamond reflects initial/primary surgery performed to build the personalized ctDNA assay, all other additional surgeries are represented with grey diamonds. (B) Distribution of pathogenic and likely pathogenic driver mutations across the 4 patients in sub-cohort B. (C) Clinical course for patient #9 who underwent an initial TAH/BSO for an ER/PR+, grade 2 uLMS, followed by cytotoxic therapy, and several metastatic resections (not depicted). Tissue from her sixth debulking procedure (denoted as additional surgery) was used to develop a personalized ctDNA assay. She subsequently received additional chemotherapy and RT (not shown) and hormonal therapy (noted in purple).