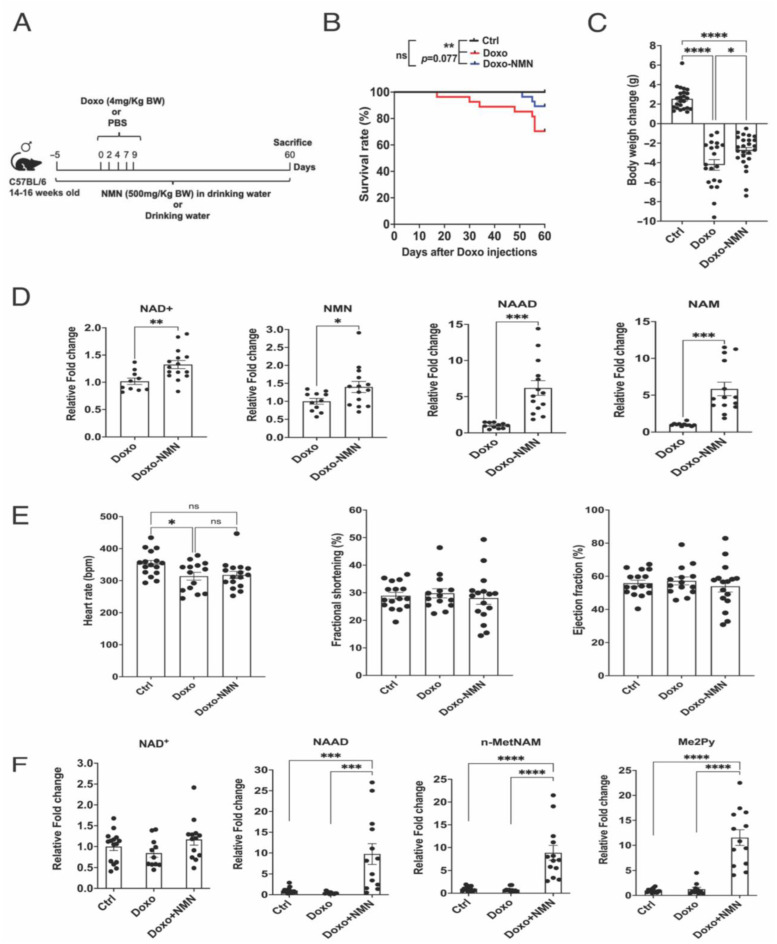
Figure 3.
Orally administered NMN is bioavailable and improves survival and body weight loss impaired by a chronic Doxo regime. (A) Study design for the chronic regimen in Doxo-induced cardiotoxicity mouse model. Preventive treatment with NMN (500 mg/kg/day, po) was initiated 5 days before five injections of Doxo (4 mg/kg/day, i.p., cumulative 20 mg/kg) and continued over 60 days. (B) Survival curve (percentage, %) of ctrl, Doxo and Doxo-NMN mice. Log-rank (Mantel–Cox) test. ** p < 0.01, ns = not significant. (C) Body weight change (grams, g) at the end of the treatment period. Body weight change has been calculated as follows: Body weight at the day of sacrifice (day 60)—body weight at the day of Doxo-injection (day 0). One-way Anova followed by Tukey’s post-test. * p < 0.05, **** p < 0.0001. (D) Levels of NAD+, NMN, NAAD and NAM (relative to Doxo group) in blood measured by LC-MS/MS. One-way Anova followed by Tukey’s post-test. * p < 0.05, ** p < 0.01, *** p < 0.001. (E) Heart rate, ejection fraction and fraction shortening were assessed by 2D-echocardiography on day 53 after the first Doxo injection. One-way Anova followed by Tukey’s post-test. * p < 0.05, ns = not significant. (F) Levels of NAD+, NAAD, n-MetNAM and Me2Py (relative to Ctrl group) in heart tissue measured by LC-MS/MS. One-way Anova followed by Tukey’s post-test. *** p < 0.001, **** p < 0.0001. Data are shown as means ± the standard error.