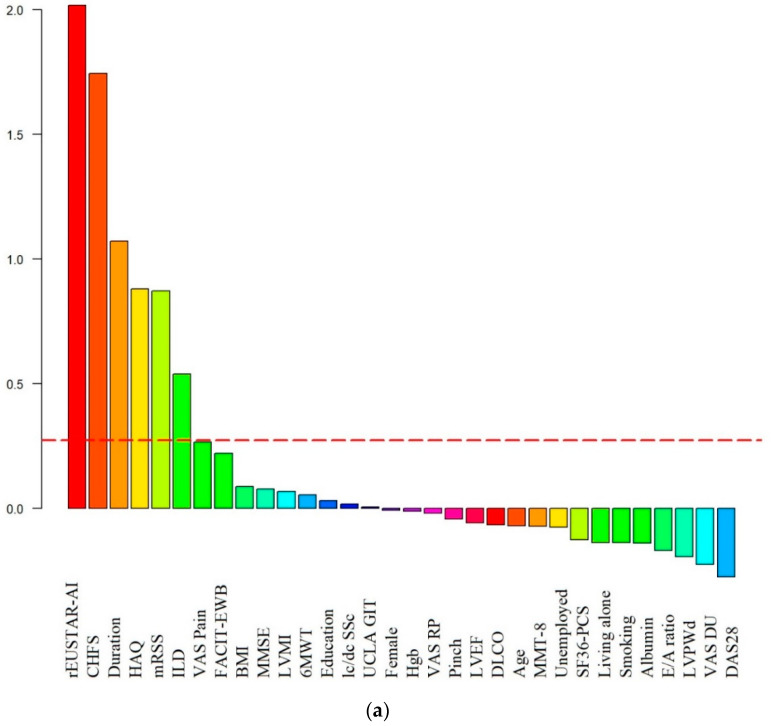
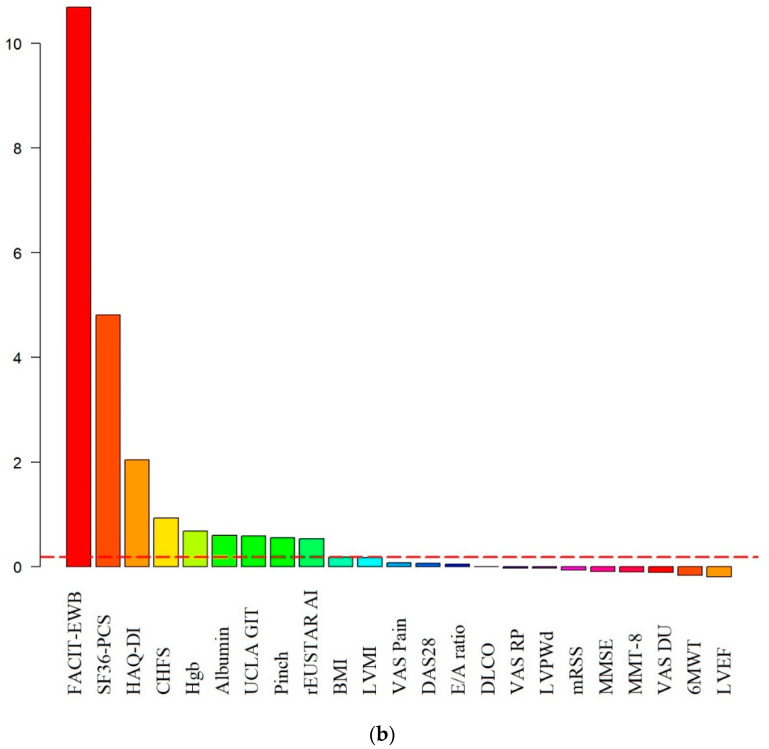
Figure 2.
Results of the Random Forest algorithm for the FACIT-FS changes to select the most important factors regarding alterations in levels of fatigue during 12-month follow-up in 144 followed patients with systemic sclerosis. (a) Potential predictors among the baseline parameters; (b) Influencing factors of the changes of the results FACIT -FS during the follow-up. Y axis represents the relative variable importance (Arbitrary unit). All variables beyond the red dashed line are considered as potential predictors. rEUSTAR AI, revised European Scleroderma Trials and Research Group Activity Index; CHFS, Cochin Hand Function Scale; Duration, Disease Duration Time; HAQ, Health Assessment Questionnaire Disability Index; mRSS, Modified Rodnan Skin Score; ILD, Interstitial Lung Disease; VAS, Visual Analogue Scale; FACIT-EWB, The Functional Assessment of Chronic Illness Therapy Emotional Well-Being; BMI, Body Mass Index; MMSE, Mini Mental State Examination; LVMI, Left Ventricular Mass Index; 6MWT, Six-minute Walk Test; lcSSc, limited cutaneous Systemic sclerosis; dcSSc, diffuse cutaneous Systemic sclerosis; UCLA GIT, University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument 2.0; Hgb, Hemoglobin; RP VAS, VAS for Raynaud’s Phenomena; Pinch, Pinch Strenght; LVEF, Left Ventricular Ejection Fraction; DLCO, Diffusing Capacity for Carbon Monoxide; MMT8; Manual Muscle Testing-8; DU VAS, VAS for Digital Ulcers; DAS28, Disease Activity Score 28; SF36-PCS, Short Form 36 Physical Component Summary; LVPWd, End-Diastolic Left Ventricular Posterior Wall thickness.