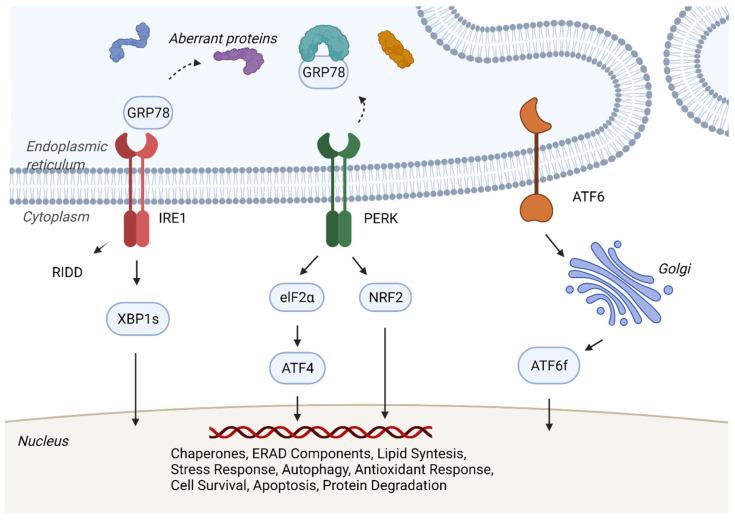
Figure 1.
Unfolded Protein Response. A simplified version of unfolded protein response (UPR). Under stress, cells activate the adaptative UPR to establish cellular homeostasis and proteostasis. The sensors IRE1, PERK, and ATF6 are activated after the dissociation of GRP78, which is recruited due to the accumulation of misfolded and unfolded proteins. IRE1 oligomerization and auto-phosphorylation induce kinase and RNase activity leading to mRNA degradation (RIDD) and altered splicing of the XBP1 mRNA and production of the transcription factor XBP1s. PERK undergoes oligomerization and auto-phosphorylation, subsequently phosphorylating the eukaryotic translation initiation factor eIF2α, resulting in the translation of transcription factor ATF4. NRF2 is also phosphorylated by PERK and activates antioxidant responses. ATF6 activation occurs after transport to the Golgi apparatus where it undergoes S1P/S2P protease cleavage, yielding transcriptionally active ATF6f. The transcriptional events mediated by XBP1s, ATF4, and ATF6f promote the expression of chaperones and components of ER-associated protein degradation (ERAD), reducing protein translation and increasing ER capacity in order to restore homeostasis. Additionally, UPR-mediated gene expression also directly impacts autophagy, cytokine production, and apoptosis. Figure 1 was created with BioRender.com.