Figure 4.
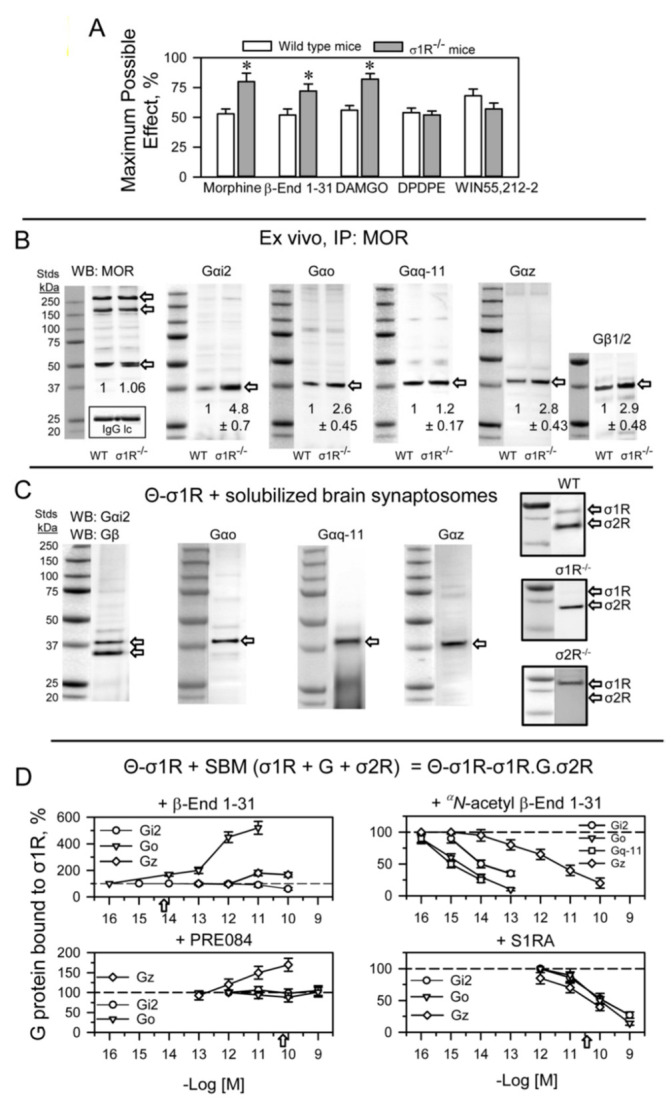
σ1R binds to G proteins and regulates MOR signaling in the mouse brain. (A) The effect of σ1R deletion on opioid- and cannabinoid-induced analgesia. Analgesic compounds were icv-injected into wild-type and σ1R−/− mice, and analgesia was evaluated 30 min after morphine (3 nmol) and β-End 1–31 (0.5 nmol), 15 min after DAMGO (0.1 nmol) and DPDPE (10 nmol) and 10 min after WIN55,212-2 (15 nmol). The bars are the mean ± SD of eight mice per strain and treatment. * Significant difference with respect to the effect of the analgesic substance in the wild-type group. ANOVA followed by the Holm–Sidak multiple comparisons test, p < 0.05, 1-β > 0.80. (B) Coprecipitation of G proteins with MORs. Solubilized mouse brain cortical membranes enriched in synaptosomes (SBM) from CD1 wild-type (WT) and σ1R−/− mice were solubilized with sonication in the presence of 1% NP-40. Non-soluble debris was eliminated, and the samples were then incubated overnight at 4 °C with affinity-purified biotinylated IgGs that were raised against extracellular regions of the MOR. Protein complexes that were immunoprecipitated with streptavidin agarose were extensively washed and then resolved by SDS-PAGE. The target G proteins, alpha and beta subunits, were visualized by Western blotting (for further details, see Section 4). IP: immunoprecipitated protein, WB: Western blot. Detection of MORs: these proteins were detected with an anti-MOR antibody directed to the C-terminal sequence, a different amino acid sequence than that used for immunoprecipitation (second extracellular loop-2EL). In parallel blots, an antibody directed to the light IgG chain of the anti-MOR antibodies used for immunoprecipitation of MORs provided a loading control for the samples in the gels. For each group, WT and σ1R−/−, the densities of the MOR-related bands indicated by the arrows, were pooled, and the computed value of the ko mice was referred to that of WT mice (assigned an arbitrary value of 1 value of 1). The immunosignals of Gα subunits coprecipitated with MORs were referred to those of the MORs. Data are the mean ± SD of three determinations. ANOVA followed by the Holm–Sidak multiple comparisons test, p < 0.05, 1-β > 0.80. (C) Pull-down assays using mouse SBM. Cerebral cortex fractions enriched in synaptosomes from CD1 WT mice were solubilized with RIPA buffer and incubated for 2 h with recombinant σ1Rs covalently attached to agarose-NHS (Θ-σ1R). Agarose pellets containing the bound proteins were resolved by SDS-PAGE, and the G protein α subunits were probed in Western blots. The capture of σ1Rs and σ2Rs by Θ-σ1R was also determined in SBM from WT, σ1R−/− and σ2R−/− mice. The assays were repeated twice, producing comparable results. Representative blots are shown. (D) Effect of ligands on the association of endogenous G proteins present in mouse SBM with Θ-σ1R oligomers produced in vitro (see Section 4). The Θ-σ1Rs were incubated with the SBM in the absence and presence of increasing concentrations of σ1R ligands. The G proteins that remained bound to Θ-σ1R oligomers were determined as in (C). The arrows indicate the affinity of the ligand for σ1Rs as determined through in vitro assays. For each compound and concentration studied data are the mean ± SD, n = 3. Data were analyzed by pairwise Holm–Sidak multiple comparison tests following ANOVA: p < 0.05, 1-β > 0.80.
