Figure 7.
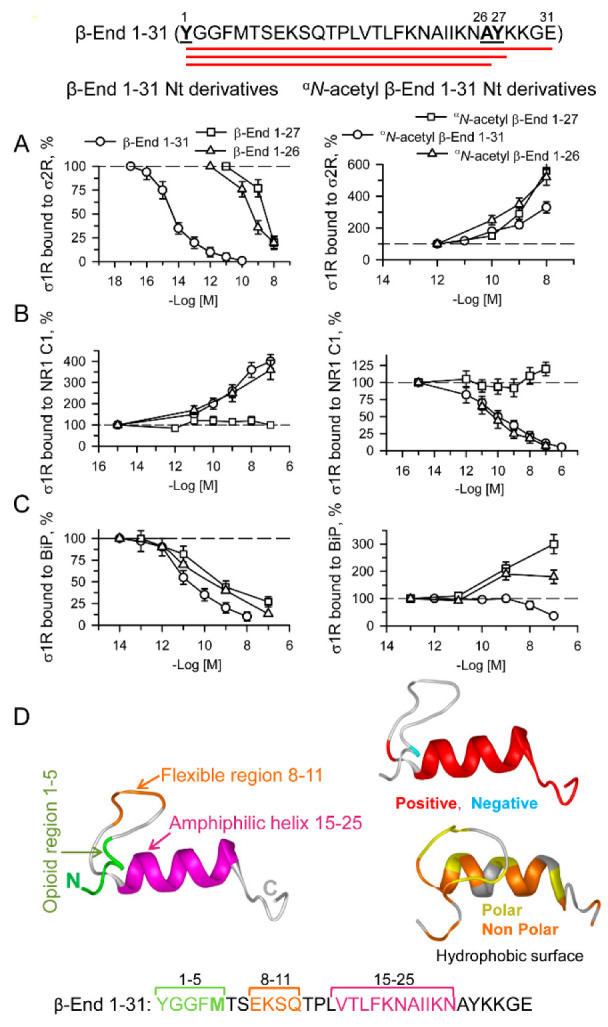
Effect of β-End 1–31, αN-acetyl β-End 1–31 and their N-terminal derivatives on σ1R-σ2R, σ1R-NR1 C1 and σ1R-BiP associations. Amino acid sequence of β-End 1–31 showing derivatives 1–26 and 1–27. (A) HaloLink-attached σ2Rs were incubated for 30 min at RT in the presence of σ1Rs (100 nM) in 300 μL of 50 mM Tris-HCl (pH 7.4), 0.2% CHAPS, and 2.5 mM CaCl2. After removing the unbound σ1R proteins, the effects of increasing the peptide concentrations on σ1R-σ2R associations were studied. At the end of the incubation, protein complexes bound to HaloLink-σ2R were obtained by centrifugation, washed three times, solubilized in 2× Laemmli buffer containing β-mercaptoethanol, resolved by SDS-PAGE, and analyzed by Western blotting (for more details, see Section 4). This protocol was also used to assess the effect of ligands on (B) σ1R-NR1 C1 and (C) σ1R-BiP associations. Data were compared to that of the control, which was obtained in the absence of ligands. (A–C) Data are the mean ± SD, n = 3. (D) The β-End 1–31 structural models were predicted by Novafold (DNASTAR Lasergene protein v.17, Inc., Madison, WI, USA). Ribbon model: The 3D structure, charge distribution and hydrophobicity map of β-End 1–31 are shown. Positive charges are shown in red and negative charges in blue. Polar surfaces are shown in yellow, and nonpolar surfaces are shown in orange. Linear model: the opioid (1–5) and flexible region (8–11) sequences are shown in green and orange, respectively, and the amphiphilic helix (15–25) is shown in pink.
