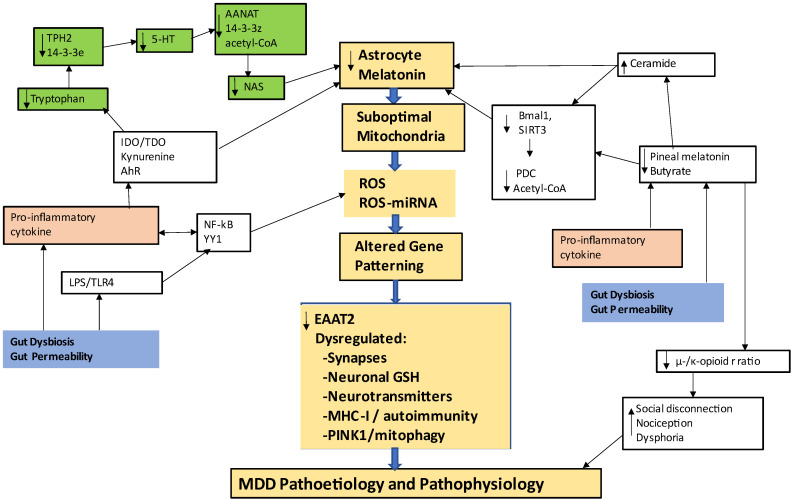
Figure 1.
Shows how an array of different processes can impact mitochondrial function, highlighting factors regulating the astrocyte mitochondrial melatonergic pathway and its consequences for MDD pathophysiology (gold shade). The tryptophan-melatonin pathway is shaded in green. Tryptophan is taken up into cells where it is converted to serotonin by tryptophan hydroxylase (TPH2). In astrocytes, TPH2 requires 14-3-3ε for stabilization. Serotonin (5-HT) is converted to N-acetylserotonin (NAS) by AANAT, which requires 14-3-3ζ for stabilization, as well as acetyl-CoA. Factors suppressing the availability of these 14-3-3 isoforms will limit melatonergic pathway induction. The AhR and ceramide can suppress 14-3-3, thereby suppressing the melatonergic pathway. The AhR, purinergic P2Y1 and metabotropic glutamate receptor (mGluR)5 can ‘backward’ convert melatonin to NAS via O-demethylation (P2Y1 and mGluR5 not shown for clarity). Gut dysbiosis and gut permeability (blue shade) increase LPS and suppress butyrate, in conjunction with increased pro-inflammatory cytokines. Pro-inflammatory cytokines and stress/cortisol increase IDO and TDO, depleting tryptophan by converting it to kynurenine, which activates the AhR as well as leading to neuroregulatory kynurenine pathway products. Pro-inflammatory cytokines and LPS also suppress pineal melatonin, contributing to the loss of the circadian ‘resetting’ of mitochondria via pineal melatonin induction of Bmal1 and, like butyrate, sirtuin-3. Bmal1 and sirtuin-3 disinhibit PDC increasing pyruvate conversion to acetyl-CoA, which is necessary to induce the melatonergic pathway. The decrease in butyrate attenuates its induction of the μ-opioid receptor, thereby impacting on immune regulation, social processes and nociception. LPS and pro-inflammatory cytokines increase inflammatory factors via the transcription factors, NF-kB and YY1, which are normally dampened by their sequential induction of melatonin via melatonin’s intracrine, autocrine and paracrine effects. The impact of the gut and circadian dysregulation thereby has consequences for mitochondrial metabolism and increased ROS, leading to alterations in patterned miRNAs and consequently in patterned gene expression. Such alterations in astrocytes change neuronal regulation and transmitter release, with differential effects in distinct brain regions genetically and epigenetically primed by developmental processes. Abbreviations: AANAT: aralkylamine N-acetyltransferase; AhR: aryl hydrocarbon receptor; ASMT: N-acetylserotonin O-methyltransferase; CYP: cytochrome P450; IDO: indoleamine 2,3-dioxygenase; LPS: lipopolysaccharide; MHC-I: major histocompatibility complex-class I; NAS: N-acetylserotonin; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; PDC: pyruvate dehydrogenase complex; PINK1: PTEN-induced kinase 1; ROS: reactive oxygen species; TDO: tryptophan 2,3-dioxygenase; TLR: Toll-like receptor; YYI: yin yang 1.