Abstract
Over the past 20 years, a variety of potential adjuvants have been studied to enhance the effect of oral vaccines in the intestinal mucosal immune system; however, no licensed adjuvant for clinical application in oral vaccines is available. In this review, we systematically updated the research progress of oral vaccine adjuvants over the past 2 decades, including biogenic adjuvants, non-biogenic adjuvants, and their multi-type composite adjuvant materials, and introduced their immune mechanisms of adjuvanticity, aiming at providing theoretical basis for developing feasible and effective adjuvants for oral vaccines. Based on these insights, we briefly discussed the challenges in the development of oral vaccine adjuvants and prospects for their future development.
Key Points
| Biogenic adjuvant materials used in the study of oral vaccines include bacteria-derived adjuvants, biologic proteins or peptides, intestinal immune cells targeting peptides, and some small-molecule immunomodulatory proteins. |
| Non-biogenic adjuvant materials used in oral vaccine studies mainly involve biodegradable polymers {such as poly(d,l-lactide-co-glycolide) [PLG], poly( d,l-lactic-co-glycolic acid) [PLGA], chitosan and their derivatives, alpha-galactosylceramide [α-GalCer], ulex europaeus agglutinin-1 [UEA-1]}, and some synthetic toll-like receptor agonists and their derivatives. |
| The combination of multitype materials has been used to design oral adjuvants; some protein vaccines (or biogenic adjuvants) are usually coated (or capsuled) with polymer-based microparticles/nanoparticles to prevent degradation in mucosa; some biogenic adjuvants are usually combined with the engineered living intestinal beneficial bacteria as a carrier to construct oral vaccine candidates. |
Introduction
Pathogens initiate infection mainly by accessing the mucosal surface of the host, especially from oral-to-gastrointestinal tract (GIT). It is generally considered that direct vaccination via the mucosal surface at the initial site of infection is the most effective way to trigger protective mucosal immune response against pathogens [1–3], but the vast majority of vaccines are administered by injection [2]. Compared with parenteral vaccination (or traditional injection), peroral vaccination or administration requires less stringent regulatory requirements, allowing for the self-administration of oral formulations. For humans, oral vaccination will minimize the need for trained healthcare personnel [4, 5] and eliminate occupational needle-stick injuries, which could reduce blood-borne infectious diseases such as acquired immune deficiency syndrome (AIDS)/human immunodeficiency virus (HIV) and hepatitis [6, 7]. For animal husbandry and aquaculture, the use of oral vaccination for disease prevention and control can reduce the labor cost of animal management and reduce the stress response of animals. Therefore, oral vaccination is potentially easier, safer, more convenient, more time-saving, and more economical [8].
However, oral vaccination is still challenging because most oral vaccinations universally could not trigger sufficient immune response, mainly because of an inadequate specific secretory immunoglobulin A (sIgA) response. There are two main reasons for this dilemma. First, the harsh conditions of the gastrointestinal environment, including hydrochloric acid, digestive enzymes, bile salts, mucus, antimicrobial peptides, and gastrointestinal peristalsis, would cause low bioavailability of antigens, which leads to acquired immune tolerance instead of stimulation [9–12]. Second, antigen-presenting cells (APCs) residing in the GIT are tolerogenic and hyporesponsive [13]; therefore, substantial impediments exist for oral vaccines to reach the inductive site of the mucosa-associated lymphoid tissues (MALT) and to trigger immune response, which critically hinders the effectiveness of oral mucosal immunization.
Given the poor immunogenicity (or low bioavailability) of oral vaccines, using appropriate and effective oral mucosal adjuvants may be critical to the success of peroral mucosal vaccination. Typical adjuvants, such as alum, complete Freund’s adjuvant (CFA), and incomplete Freund’s adjuvant (IFA), etc., have long been used in injectable vaccination but they do not work well in peroral mucosal immunity. To successfully stimulate intestinal mucosal immunity, oral vaccine adjuvants need to have two main properties—GIT delivery stability and intestinal mucosal adjuvanticity—because they must find an effective way to deliver vaccines (or antigens) to the dendritic cells (DCs), macrophages, and lymphocytes located in MALT through these natural barriers in GIT, and exert their adjuvant properties, while protecting the loaded vaccines (or antigens) from the harsh peroral mucosal environment. Therefore, it is necessary to explore effective peroral mucosal adjuvants to improve the effectiveness of oral vaccines; however, to date, no adjuvants have been included in licensed oral vaccines [14].
In recent years, many researchers have focused on finding safe and effective adjuvants (or delivery systems) to formulate oral vaccines, and have made great progress in the development of oral vaccine adjuvants. At present, some potential oral mucosal adjuvants have shown promising prospects, for instance modified bacterial enterotoxins (e.g., double-mutant heat-labile toxin [dmLT] of Escherichia coli and multiple mutant cholera toxin [mmCT]), some small molecule immunomodulatory proteins, and some non-biogenic biodegradable polymer materials {e.g., poly(d,l-lactide-co-glycolide) [PLG], poly(d,l-lactic-co-glycolic acid) [PLGA], alpha-galactosylceramide [α-GalCer], Ulex europaeus agglutinin-1 [UEA-1]}, and some synthetic toll-like receptor (TLR) agonists and their derivatives.
In this review, we systematically summarize various peroral adjuvant candidates, including the completed, ongoing, and planned study candidates. According to the physicochemical properties of these peroral adjuvant candidates, we classified the existing or emerging oral vaccine adjuvants as biogenic adjuvants (including bacteria-derived or biologic protein, peptide, or immunostimulants, small-molecule proteins, etc.), non-biogenic adjuvants (e.g., various biocompatible polymer-based microparticles/nanoparticles), and biogenic and non-biogenic composite adjuvants. We also introduced their general properties, mechanisms of adjuvanticity, origins and brief histories, preparation processes, and results of preclinical studies or even clinical studies, and discussed prospects for their application as oral vaccine adjuvants. This article reviews the research progress of oral adjuvants in recent years, aiming to promote the application prospects of oral vaccines.
Biogenic Type Oral Vaccine Adjuvants
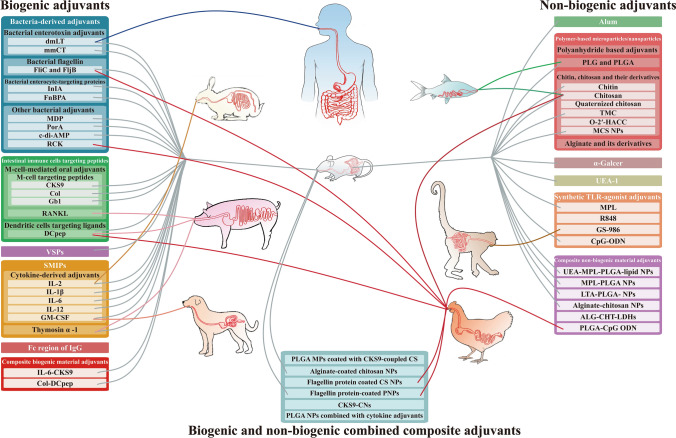
At present, many effective oral vaccine adjuvants are still derived from biological material, such as bacteria-derived adjuvants (e.g., bacterial enterotoxins, bacterial flagellin, bacteria-derived enterocyte-targeting proteins, and some bacteria-derived proteins), protozoan-derived adjuvants, intestinal immune cells targeting peptide adjuvants, small molecular immunomodulatory proteins (SMIPs; e.g., cytokines and thymosin α-1 [Tα1]), Fc region of immunoglobulin (Ig) G, and adjuvants composed of multiple biogenic materials (Table 1).
Table 1.
Current developments in biogenic adjuvants for oral administration vaccines
| Adjuvant name | Explanation | Disease model (pathogen) | Selected antigen or vaccine (or candidate) formulation | Animal model or clinical trials | Results of immune response in oral administration trials | Reference |
|---|---|---|---|---|---|---|
| Bacterial-derived oral vaccine adjuvants | ||||||
| Bacterial enterotoxins | ||||||
| dmLT | Double mutant (R192G L211A) heat-labile toxin from ETEC | Bacterial diarrhea in children and travelers (ETEC) | Oral vaccination with EtpA and dmLT | CD-1 mice |
Significant protection against small intestinal colonization of ETEC strain The degree of protection correlated with fecal IgG, IgA, or total fecal antibody responses to EtpA |
[199] |
| dmLT | As above | Bacterial diarrhea in children and travelers (ETEC) | Orally administered with an attenuated ETEC vaccine candidate (ACE527) and dmLT | Human trial |
Challenge strain shedding was tenfold lower in those receiving the adjuvant than those receiving vaccine alone The unadjuvanted vaccine was not protective 83% showed significant mucosal IgA responses Significantly increased intestine-derived anti-CS6 responses compared with vaccine alone |
[28–30] |
| dmLT | As above | Bacterial diarrhea in children and travelers (ETEC) | Orally administered with an oral ETEC vaccine (ETVAX) and dmLT | Phase I/II trial in Bangladesh | Enhanced the magnitude, breadth, and kinetics of immune responses in infants | [187, 188] |
| dmLT | As above | H. pylori infection | Orally administered with H. pylori lysate antigens and dmLT | C57BL/6 mice |
Significant decrease in bacterial load compared with the unimmunized controls The same extent as CT as an adjuvant Enhanced in vitro proliferative and cytokine responses to H. pylori antigens |
[200] |
| dmLT | As above | H. pylori infection | Oral immunization with recombinant H. pylori protein antigens (NAP/UreA/UreB) and dmLT | BALB/c mice |
Enhanced antigen-specific lymphocyte proliferation, and serum IgG and mucosal IgA responses Increased the proportion of CD4+ IL-17+ lymphocytes Enhanced the production of IL-17, IL-16, IL-6 and TNFα Confers more effective prophylactic protection against H. pylori infection |
[201] |
| LTB | Escherichia coli heat-labile enterotoxin B subunit protein | H5N1 HPAI | H5N1 chimeric VLPs composed of the viral HA, NA, and M1 proteins and LTB | BALB/c mice |
Conferred substantial protection against lethal challenge Showed tenfold higher virus-specific IgG titers than mice immunized with H5N1-VLPs lacking LTB |
[39] |
| CT | Cholera toxin | Necrotic enteritis (Clostridium perfringens type A) | Orally administered live vaccine (non-virulent NetB-producing strain of C. perfringens) and CT | Broiler chickens | 55% of vaccinated birds did not develop any lesions of NE after challenge, compared with 100% incidence in the unvaccinated group | [202] |
| mmCT | Multiple mutant cholera toxin | H. pylori infection | Intragastric immunizations with formalin-inactivated H. pylori whole cell vaccine admixed with mmCT | C57BL/6 mice |
50- to 125-fold reduction in colonization of H. pylori Rise in both serum IgG and intestinal mucosal IgA anti-H. pylori antibody responses Strong T cell and IFNγ and IL-17A cytokine responses |
[32] |
| Bacterial flagellin | ||||||
| FliC | Salmonella enterica serovar Typhimurium flagellin |
H5N1 HPAI |
H5N1 chimeric VLPs composed of the viral HA, NA, and M1 proteins and FliC | BALB/c mice |
Conferred substantial protection against lethal challenge Showed tenfold higher virus-specific IgG titers than mice immunized with H5N1-VLPs lacking FliC |
[39] |
| FliC and FljB flagellins | Diphasic S. typhimurium possesses two flagellins (phase I flagellin FliC and phase II flagellin FljB) | Salmonellosis | The recombinant attenuated S. typhimurium BRD509 strain (the iacP mutantstrain) expressing FliC and FljB flagellins | BALB/c mice | Strongly enhanced NF-κB activation and proinflammatory cytokine expression in vitro and ex vivo | [42] |
| Flagellin | Salmonella enterica subsp. flagellin | Rabies viruses | Recombinant rabies viruses expressing flagellin (LBNSE-Flagellin) | ICR mice |
LBNSE-Flagellin is more immunogenic than the parent virus Induce greater activation and maturation of DCs and B cells |
[34] |
| Flagellin | S. typhimurium flagellin | Fowl typhoid (Salmonella gallinarum) | S. gallinarum ghosts expressing S. typhimurium flagellin | Female layer brown nick chicks |
Improved antigen-specific humoral and cell-mediated immune responses Enhanced protective efficacy against the virulent challenges |
[41] |
| Flagellin | Salmonella flagellin | HBV | A live recombinant Salmonella dublin vaccine strain expressing HBsAg epitopes inserted in the hypervariable region of a cloned Salmonella flagellin gene | Guinea pigs and mice | Developed detectable antibodies specific to HBV epitopes | [45] |
| Flagellin | Salmonella typhimurium flagellin | V. cholerae | A flagellin-negative S. dublin strain expressing the chimeric Salmonella flagellin gene inserted with cholera toxin B epitopes | C57BL/6 mice | Produced high levels of antibody against cholera toxin | [46] |
| FliCd | Salmonella enterica FliCd flagellin | Plasmodium yoelii | A live S. dublin vaccine strain expressing the target CS280–288 peptide fused at the central hypervariable domain | BALB/c mice | Primed CS280–288-specific cytotoxic CD8+ T cells | [47] |
| Bacterial-derived enterocyte cell-targeting proteins | ||||||
| mInIA | The mutated form of internalin A of L. monocytogenes | Cow’s milk allergy (bovine β-lactoglobulin) | L. lactis expressing mInIA and transformed with pValacBLG | BALB/c mice |
The plasmid transfer in vitro was increased 10 times The number of mice producing BLG was slightly higher |
[50] |
| FnBPA | Fibronectin-binding protein A of S. aureus | NM | Recombinant invasive L. plantarum expressing FnBPA | C57BL/6 and BALB/c mice |
Invasion ratios of L. plantarum strain on the IPEC-J2 cell line was about twofold that of the empty vector Induced specific humoral immune response |
[51] |
| FnBPA | As above | Cow’s milk allergy (bovine β-lactoglobulin) | L. lactis expressing FnBPA and carrying the plasmid pValac:BLG (LL-FnBPA+ BLG) | BALB/c mice |
Co-incubated with LL-FnBPA+ BLG produced up to 30 times more BLG LL-FnBPA+ increased the number of mice producing BLG |
[52] |
| FnBPA | As above | Tuberculosis (M. tuberculosis) | L. lactis FnBPA+ strain carrying the eukaryotic expression vector coding the ESAT-6 gene of M. tuberculosis | BALB/c mice |
Significantly increased IFNγ production Significant increase in specific sIgA production |
[53] |
| Additional bacteria-derived adjuvants | ||||||
| MT | Muramyl dipeptide and tuftsin fusion protein | Transmissible gastroenteritis virus | L. casei expressing the MDP and tuftsin fusion protein repeated 20 and 40 times, and the D antigenic site of the spike protein of TGEV | BALB/c mice | Enhanced the anti-TGEV antibody immune responses of both humoral and T cell-mediated immune systems | [54] |
| PorA | An OMP of N. meningitidis | H. pylori | L. lactis strain expressing a PorA-HpaA hybrid | BALB/c mice | Enhanced the antibody response against the HpaA antigen approximately threefold | [57] |
| c-di-AMP | A bacterial second messenger | T. cruzi parasite | L. lactis strain expressing antigenic TScf combined with another L. lactis strain producing c-di-AMP | BALB/c mice | Elicit a TS-specific immune response | [58] |
| RCK | An OMP of Salmonella enterica | IBDV | A recombinant L. lactis co-expressing the major IBDV antigens VP2 and RCK protein | Chickens |
Induced a specific neutralizing antibody-mediated immune response Conferred full protection against very-virulent IBDV challenge |
[62] |
| Protozoan-derived adjuvants | ||||||
| VSPs | Variant-specific surface proteins covered on the Giardia lamblia surface | Influenza A (H5N1 or H1N1) | Chimeric VLPs decorated with VSPs and expressing influenza virus HA and NA | BALB/c, C57BL/6, C57BL/10ScNJ (TLR-4 KO) mice |
Protected antigens from degradation and enhanced their immunogenicity Generated robust immune responses that protect mice from influenza infection and HA-expressing tumors |
[63] |
| Intestinal immune cell-targeting peptide adjuvants | ||||||
| M cell-targeting adjuvants | ||||||
| Co1 | M cell-specific peptide ligand | EGFP as model Ag | Co1-fused EGFP proteins | BALB/c mice |
Co1-fused EGFP binds to M cells Transported effectively into the mucosal immune induction site |
[78] |
| Co1 | M cell-targeting peptide | PED (PEDV) | Genetically engineered L. casei 393 strain fused expressing PEDV COE antigen and M cell-targeting peptide Co1 (pPG-COE-Co1/L393) | BALB/c mice | Effectively induce mucosal, humoral, and Th2-type cellular immune responses against PEDV infection | [79] |
| CKS9 | M cell-targeting peptide ligand (CKSTHPLSC) | Swine dysentery (Brachyspira hyodysenteriae) | Cytoplasmic expression of a model antigen (BmpB) with M cell-targeting moiety in a recombinant L. plantarum strain | BALB/c mice |
CKS9 could efficiently deliver its conjugated BmpB from the intestinal lumen into GALT via M cells Induce strong mucosal and systemic immune responses against BmpB |
[80] |
| Gb-1 | GP-2 (an integral membrane protein expressed specifically on M cells) binding peptides | EGFP as model Ag | Gb1-EGFP fusion proteins | BALB/c mice |
Gb-1 showed high binding affinity to GP-2 Significantly increased the uptake of EGFP by M cells Induced efficient mucosal and systemic immune responses Induced a Th2-type immune response |
[82] |
| Cytokine receptor activator of NF-kB ligand (RANKL) | ||||||
| RANKL | Cytokine receptor activator of NF-kB ligand | Porcine epidemic diarrhea (PEDV) | Oral vaccination with aP2 subunit vaccine-loaded HPMCP and RANKL-secreting L. lactis (HPMCP [aP2] plus LL RANKL) | Pregnant sows |
Significantly increased titers of virus-specific IgA antibodies and neutralizing antibodies The survival rate of piglets delivered by sows vaccinated with HPMCP (aP2) plus LL RANKL was similar to those vaccinated with a commercial killed PEDV vaccine |
[86] |
| DC-targeting adjuvants | ||||||
| DCpep | DC-targeting peptide (FYPSYHSTPQRP) | Bacillus anthracis infections | L. acidophilus expressing a PA-DCpep fusion | A/J mice |
Induced robust protective immunity against lethal challenge of B. anthracis The serum anti-PA titers, neutralizing PA antibodies, and the levels of IgA-expressing cells were comparable with the recombinant PA plus aluminum hydroxide vaccine administered SC |
[70] |
| DCpep | As above | M. tuberculosis | Recombinant L. plantarum secreting and anchoring of M. tuberculosis antigens (Ag85B-ESAT6) fused with DCpep | Mice | The pro-inflammatory cytokines (IFNy and IL-17A) increased | [89] |
| DCpep | DC-targeting peptide | PED (PEDV) | A recombinant L. casei expressing a DC-targeting peptide fused with the PEDV core neutralizing epitope antigen | BALB/c mice, large white piglets |
Lactobacillus vaccine elicits a specific systemic and mucosal immune response Promotes lymphocyte proliferation Effectively protects piglets against PEDV infection |
[90, 91] |
| DCpep | DC-targeting peptide | H9N2 AIVs | Recombinant L. plantarum NC8 expressing HA and DCpep | BALB/c mice and white leghorn chickens |
Elicited high serum titers of hemagglutination-inhibition antibodies in mice Induced robust T-cell immune responses in both mouse and chicken models |
[92] |
| SP |
Chicken DC-binding peptides Candidate (SPHLHTSSPWER) |
IBDV |
Recombinant L. saerimneri M11 delivering IBDV structural protein and protective antigen VP2 fused with SP |
SPF chickens |
Efficiently induced anti-IBDV mucosal and humoral immune responses Resulting in higher protective efficacy in the VP2-SP group than the VP2 group |
[197] |
| SMIPs | ||||||
| Cytokine adjuvants | ||||||
| IL-1β | Murine IL-1β | Enteritidis (Salmonella enterica) | Intragastric immunization with heat-killed Salmonella enterica in combination with recombinant L. casei producing IL-1β | BALB/c mice and C3H/HeJ mice | Resulted in relatively high Salmonella enterica-specific antibody production | [95] |
| IL-1β fragment | A fragment of human IL-1β (VQGEESNDK peptide) | Clostridium difficile | Recombinant Bacillus subtilis spores presenting a chimeric Protein (C. difficile FliD protein and an IL-1β fragment) | BALB/c mice | Significantly changed the characteristics of elicited immune response | [96] |
| IL-2 | Human IL-2 | H. pylori infection | Recombinant L. lactis NZ9000 containing a common immunogen of H. pylori (UreB) as a chimeric protein fused with human IL-2 | BALB/c mice |
Elicited more anti-UreB antibody and more cytokines Had a lower H. pylori burden and urease activity than control mice |
[98] |
| IL-2 | Human IL-2 | H. pylori | Coadministered with recombinant B. subtilis spores expressing the Helicobacter acinonychis UreB protein and another B. subtilis spore presenting IL-2 | BALB/c mice | Elicited a strong cellular immune response | [99] |
| IL-2 | Rabbit IL-2 | Rabbit hemorrhagic disease | A DNA vaccine co-expressing IL-2 and VP60 and delivered by attenuated Salmonella typhimurium | Chinese white rabbits |
Induced a higher level of antibodies to a significant extent Concentrations of IL-4 were markedly higher The fusion gene vaccine provided higher protection |
[97] |
| IL-12 | Mouse IL-12 | Leishmania major infection | L. lactis(alr-) co-expressing the protective Leishmania antigen (secLACK) and mouse secIL-12 | BALB/c mice |
Generated protective immunity against Leishmania major infections Induced Th1 immune response mediated by CD4+ T cells |
[100] |
| cGM-CSF | Canine GM-CSF | Canine corona virus | Coadministration CCV vaccine and L. lactis expressing cGM-CSF | Beagle puppies |
Monocyte counts in hematology and serum IgA were higher Increased more CCV-specific IgG in serum |
[101] |
| Tα1 | Non-toxic immune-modifier peptide hormone from the thymus | CSFV | Recombinant L. plantarum bacteria expressing CSFV E2 protein in conjunction with Tα1 (L. plantarum/pYG-E2-Tα1) | Pigs |
Effectively induced protective immune responses in pigs against CSFV infection Significant differences in the levels of immune responses between L. plantarum/pYG-E2-Tα1 and L. plantarum/pYG-E2 |
[102] |
| Fc region of IgG and Anti-DEC-205 antibody | ||||||
| IgG Fc fragment | Fc fragment of mouse IgG2a | Influenza virus (H1N1 and H9N2) | Recombinant L. plantarum expressing the internal influenza viral protein M2e fused to an IgG Fc fragment | BALB/c mice |
Markedly reduced the viral load in the lungs Protected against H1N1 influenza virus and mouse-adapted H9N2 AIV challenge |
[112] |
| Fc fragments conjugated to nanoparticles | Fc fragment of IgG antibodies | Diabetes | Fc-targeted nanoparticles encapsulating insulin (insNP-Fc) | Wild-type mice and FcRn knockout mice |
FcRn-targeted nanoparticles crossed the intestinal epithelium and reached systemic circulation with a mean absorption efficiency of 13.7%*h Elicited a prolonged hypoglycemic response in wild-type mice |
[203] |
| aDec | Anti-DEC-205 antibody | NM | An engineered L. plantarum strain expressing aDec and containing a plasmid for expression of GFP under control of a eukaryotic promoter | C57BL/6 mice |
DCs showed increased uptake of the engineered L. plantarum Increased internalization of L. plantarum and plasmid transfer in DCs |
[204] |
| Multiple biogenic oral adjuvants | ||||||
| DCpep and Co1 | DC and M cell-targeting peptides | PED (PEDV) | Recombinant L. casei 393 strain expressing DCpep and Co1-targeting ligands fused with the PEDV COE antigen | BALB/c mice | Promoted stronger, more rapid antigen-specific immune responses | [114] |
| IL-6-CKS9 | A recombinant cytokine conjugating an M cell-targeting peptide (CKS9) with C-terminus of the murine IL-6 | Swine dysentery (B. hyodysenteriae) | M-BmpB protein combined with the recombinant L. lactis IL-1403 producing IL-6-CKS9 | BALB/c mice |
Induced both Th1- and Th2-type immune responses Enhanced induction of antigen-specific antibody in both mucosal and systemic immune response |
[75] |
The immune response results of the above examples are all the results of the oral immunization test
aDec anti-Dec-205 recombinant antibody, Ag antigen, AIV avian influenza virus, BLG β-lactoglobulin, BmpB Brachyspira membrane protein B, CCV canine corona virus, Co1 M cell-specific peptide ligands, CSFV classical swine fever virus, DC dendritic cell, EGFP enhanced green fluorescence protein, ETEC enterotoxigenic Escherichia coli, ETVAX an oral, inactivated, enterotoxigenic E. coli vaccine, GALT gut-associated lymphoid tissue, GM-CSF granulocyte-macrophage colony-stimulating factor, H5N1-VLPs VLPs containing only HA, NA and M1, HA hemagglutinin, HBsAg hepatitis B surface antigen, HBV hepatitis B virus, HPAI highly pathogenic avian influenza, HPMCP hydroxypropyl methylcellulose phthalate microspheres, IBDV infectious bursal disease virus, Ig immunoglobulin, IFN interferon, IL interleukin, LACK Leishmania homolog of activated C kinase, LBNSE a recombinant rabies virus, M-BmpB Brachyspira membrane protein B conjugated with CKS9, NA neuraminidase, NE necrotic enteritis, NetB necrotic enteritis toxin B-like, NF-κB nuclear factor Kappa B, NM not mentioned, OMP outer membrane protein, PA a B. anthracis protective antigen, PED porcine epidemic diarrhea, PEDV porcine epidemic diarrhea virus, PEDV COE core neutralizing epitope (COE) of the PEDV spike protein, RANKL receptor activator of NF-kB ligand, RCK Salmonella resistance to complement killing, SC subcutaneous, sIGA secretory immunoglobulin A, SMIPs small molecular immunomodulatory proteins, Tα1 thymosin α-1, TGEV transmissible gastroenteritis virus, Th T helper, TLR toll-like receptor ligand, TNF tumor necrosis factor, VLPs virus-like particles
Bacteria-Derived Adjuvants for Oral Vaccines
Targeting specific bacterial organelles or components, the host’s immune system has evolved to recognize infections and activate the most potent immune cells to fight the pathogenic bacteria. When developing vaccines, adding appropriate bacterial organelles or components into vaccines would produce a stronger immune response to provide better and more enduring immune protection against infections. Bacteria-derived adjuvants have attracted particular interest for the development of oral vaccines because specific bacterial organelles or components have a role as immune stimulators.
Bacterial Enterotoxin Adjuvants
The most well-studied mucosal adjuvants to date are still the adenosine diphosphate (ADP)-ribosylating bacterial enterotoxins, such as cholera toxin (CT) produced by Vibrio cholerae, heat-labile toxins (LT) produced by enterotoxigenic Escherichia coli (ETEC), as well as their mutants or subunits. Initially, CT and LT were not only highly effective mucosal adjuvants but they were also very toxic, which precluded their clinical application. However, much effort has been devoted to developing variants of these enterotoxins that are low or non-toxic but still retain their adjuvant activity [15], such as LT (R192G or single-mutant LT [mLT]) [16], dmLT [17], and mmCT [18]. These enterotoxins (CT and LT) and their mutants (or subunits) [dmLT and mLT] can increase the generation of antigen-specific IgA antibodies, T-cell responses, and long-lasting memory when coadministered with antigens through the mucosal or transcutaneous routes [16, 19]. CT, LT, and some LT mutants could increase antigen capture in the small intestine by promoting DC migration from the subepithelial dome (SED) to the follicle-associated epithelium (FAE) between 1.5 and 12 h after oral administration [20]. Additionally, preclinical research showed that LT, dmLT, CT, and mmCT can all significantly raise T helper (Th) 17 responses and thus increase antibody responses (Fig. 1a) [16, 18, 19, 21].
Fig. 1.
Concise mechanisms of oral adjuvants at intestinal mucosal sites. a Bacterial enterotoxin (dmLT, mmCT) targets GM1 receptors, promotes Th17 response, and subsequently induces antigen-specific IgA antibodies. b Bacterial flagellin increases TLR5 stimulation that activates the production of inflammatory cytokines and subsequently augments innate and adaptive immune responses. c1 M cell-targeting peptides (CKS9, Co1) specifically target and bind to M cells. c2 RANKL, increasing the number of M cells. c3 DC-targeting ligand (DCpep), specifically targets and binds to dendritic cells. d Small molecular immunomodulatory proteins (cytokines and Tα1) directly stimulate, attract immune cells, and induce immune response. e1 PLG and PLGA protect antigens from degradation in GIT, allow the sustained and extended release of encapsulated antigens, and enhance antigen uptake by APCs, and subsequently the delivery of these microparticle-containing APCs to specific lymphoid compartments. e2 CS and its derivatives (TMC, HACC) and e3 PAHs possess mucoadhesive properties and permeation-enhancing effects. e4 ALG possesses mucoadhesive properties. f UEA-1 specifically targets and binds to M cells. g α-GalCer activates the iNKT-cell. h CpG-ODN activates TLR9 on B-lymphocytes and DCs, stimulates antigen presentation and induction of antigen-specific immune response towards the Th1 phenotype. CS chitosan, PAHs polyanhydrides, ALG alginate, iNKT-cell invariant natural killer T cell
Double mutant heat-labile enterotoxin (dmLT) The most widely used and promising bacterial enterotoxin adjuvant to date is LT (R192G/L211A) or dmLT. In fact, dmLT is a genetically attenuated derivative of a wild-type ETEC heat-labile enterotoxin, which changes arginine to glycine at amino acid position 192 to disrupt the enzymatic and toxic activity of LT, and changes leucine to alanine at a potential pepsin-sensitive proteolytic cleavage site at amino acid position 211 [17]. This detoxified or attenuated form of LT retains its antigenicity and adjuvant properties. dmLT has been shown to be safe, well tolerated, and reasonably immunogenic in oral doses up to 100 μg in humans [22]. To date, dmLT has been an effective adjuvant that strongly potentiated the immune responses of various vaccines administered parenterally and mucosally against infectious pathogens (Table 1), e.g., Streptococcus pneumoniae [23], Helicobacter pylori [24, 25], tetanus toxoid [17], CT [18], and ETEC [26]. Noteworthy, when prophylactic immunization was performed with H. pylori lysate antigens, dmLT promoted strong B- and T-cell immune responses to H. pylori antigens and reduced the bacterial load in stomachs of H. pylori-infected mice [25]. Adding dmLT to an attenuated Salmonella-vectored ETEC vaccine improved its immunogenicity in mice [27]. Through preclinical studies, Holmgren et al. showed that adding dmLT to the multivalent ETEC vaccine (ETVAX) significantly improved both the anti-colonization factor (CF) and anti-LT responses following oral immunization [28]. Moreover, the phase I study of human volunteer trials proved that dmLT further enhanced the mucosal immune responses to CF antigens present in low amounts in this ETVAX vaccine [28, 29]. In addition, through clinical trials of human volunteers, Harro et al. demonstrated that the shedding of challenge strain (ETEC H10407) in those human volunteers orally administered ACE527 (the ETEC vaccine) and dmLT was tenfold lower than in those who received the vaccine alone, illustrating that dmLT can significantly contribute to vaccine efficacy to protect human volunteers against ETEC challenge [30]. In conclusion, dmLT is a well-tolerated and powerful mucosal adjuvant for coadministered antigens.
Multiple Mutant Cholera Toxin (mmCT) CT used to be an effective adjuvant, widely used to induce mucosal immune responses in animal models; however, the strong enterotoxicity of CT precludes its use in human or veterinary vaccines. The recently developed mmCT, which derived from CT with mutations in multi-sites in its A subunit and is fully resistant to proteolytic cleavage, is a strong, yet practically non-toxic novel mucosal adjuvant. Compared with native CT, the cAMP-inducing activity of mmCT decreased by >1000-fold [31]. Compared with dmLT, mmCT protein is more easily produced and purified in large quantities because mmCT is secreted from the extracellular medium of CT-deleted V. cholerae, while dmLT is located in inclusion bodies [19, 31]. mmCT possesses similar adjuvant activity and safety as dmLT, which promotes human Th17 responses via cAMP-dependent protein kinase A and caspase-1/inflammasome-dependent interleukin (IL)-1 signaling [18]. The study by Holmgren et al. [32] reported that intragastric immunization of H. pylori whole-cell vaccine (WCV) together with mmCT reduced the colonization of H. pylori in the stomach of mice by 50- to 125-fold, which was associated with rises in both the anti-H. pylori antibody responses of serum IgG and intestinal mucosal IgA and the responses of strong T cell and interferon (IFN)-γ and IL-17A cytokines. Moreover, its immune effect is similar to that of WCV together with CT, indicating mmCT, a non-toxic adjuvant, can replace CT as an adjuvant without loss in protective efficacy [32].
In conclusion, mmCT has no enterotoxicity but retains strong adjuvant activity, is economical and easy to be produced, and has great potential in designing oral vaccines.
Bacterial Flagellin
Flagellin, the main structural protein of bacterial flagella, is considered a pathogen-associated molecular pattern (PAMP). TLR5 can recognize flagellin, thus activating the production of inflammatory molecules, including chemokines and cytokines (Fig. 1b), and then triggering cellular immune responses, including DCs, through myeloid differentiation factor 88 (MyD88) signaling [33, 34]. In addition to TLR5 activation, flagellin can bind to cytosolic nucleotide binding oligomerization domain-like receptors, NLRC4, which activate the caspase-1 inflammasome [35]. TLR5 is extensively expressed in the lung, intestinal epithelial cells, monocytes/macrophages, and DCs [36], Because flagellin is easy to express, is stable and potently activates the adaptive immune response by binding to TLR5 [37, 38], it has attracted a lot of attention as a vaccine adjuvant. Oral administration of flagellin-based vaccines could induce effective immune protection in mice. In the study by Ren et al. [39], the H5N1 chimeric virus-like particles (VLPs) containing membrane-anchored FliC (FliC-VLP) were administered orally to mice, and the virus-specific IgG titers of immunized mice were tenfold higher than those of mice immunized with H5N1-VLPs lacking FliC, which significantly improved the protective immune response to lethal challenge from both homologous and heterologous H5N1 viruses. According to Zhou et al. [34], mice orally inoculated with LBNSE-Flagellin (the recombinant rabies viruses [rRABV] expressing flagellin of Salmonella enterica subsp.) could recruit/activate more DCs and B cells in the periphery, and trigger a stronger adaptive immune response (i.e., virus-neutralizing antibody level). LBNSE-Flagellin could shield more mice from LD50 challenge infection with rabies viruses strain CVS-24 compared with the parent virus LBNSE group. An innovative study by Girard et al. [40] revealed that plant-produced flagellin (flagellin of Salmonella typhimurium [FljB]) was a more potent and effective adjuvant for oral immunization. Beyond that, using plant-produced flagellin as an adjuvant for oral vaccine did not elicit an immune response against FljB.
By incorporating membrane-anchored flagellin into bacterial ghosts (BGs), it may be possible to create a more effective oral BG-based vaccine [41]. Moreover, synthesizing varied flagellins in an oral live bacterial vaccine strain is an attractive method for generating protective immunity. In the study by Eom et al., mice were shown to be protected against the virulent Salmonella SL1344 strain [42] after receiving an oral immunization with attenuated S. typhimurium BRD509 vaccine strain that expressed FliC and FljB flagellins (diphasic S. typhimurium has two flagellin genes—the flagellin in phase I is FliC and the flagellin in phase II is FljB [43]). In addition, it is another promising way to develop oral probiotic live vaccine strains or oral attenuated (or non-virulent) salmonella live vaccine strains by integrating heterogeneous antigens into the hypervariable region of flagellin of probiotic strain [44] or attenuated (or non-virulent) salmonella strain [45–47].
In summary, it is commonly acknowledged that flagellin can boost an antigen-specific immune response when used as an adjuvant. This will facilitate the development of flagellin-based vaccines that are safer and more effective, as well as their entry into oral clinical trials.
Bacteria-Derived Enterocyte Targeting Proteins
Expression of enterocyte binding proteins derived from some pathogenic bacteria on the surface of probiotic strains as adjuvants to deliver eukaryotic expression plasmids into host intestinal epithelial cells could be an effective oral DNA vaccine strategy [36]. Internalin A (InIA) in Listeria monocytogenes (L. monocytogenes) and fibronectin binding protein A (FnBPA) in Staphylococcus aureus (S. aureus) are well-known enterocyte targeting proteins. InlA is a cell wall protein that allows L. monocytogenes to bind to and be internalized by epithelial cells [48]. And FnBPA is an epithelial cell binding protein that can bind to fibrinogen, elastin, and fibronectin allowing for internalization of S. aureus into non-phagocytic cells [49]. When InlA (or mInlA, the mutated form of InlA [Ser192Asn and Tyr369Ser]) [50] and/or FnBPA [51–53] were expressed on the surface of lactic acid bacteria (LAB) strains, these recombinant strains acquire the ability to invade mammalian cells through the interaction between InlA and/or FnBPA and cellular receptors, resulting in the increase of targeted antigens cDNA in the intestinal lumen and the enhancement of host immune response [50–53].
Other Bacteria-Derived Proteins
Rarely investigated as mucosal adjuvants, some bacterial proteins and messengers still lack a clear understanding of how exactly they trigger immunity. However, they could be candidates for oral vaccine adjuvants because of their capacity to facilitate the immune response to antigens.
Muramyl dipeptide (MDP) is part of the bacterial cell wall and is delivered as a dipeptide with tuftsin, a biologically active compound [54, 55]. Although their roles in oral immune adjustment have not been fully elucidated, it has been demonstrated that MDP and tuftsin can activate APCs [55]. In the study by Jiang et al. [54], the fusion protein of MDP and tuftsin was utilized as an adjuvant to modify the Lactobacillus casei vaccine strain. The results showed that antibody and T-cell responses were improved after oral administration in BALB/c mice.
PorA is an outer membrane protein (OMP) from the Neisseria meningitidis [56]. It is remarkable that PorA has an important feature of oral protein adjuvants, namely resistance to proteolytic enzymes in the GIT [57]. It has the potential to act as an oral adjuvant when conjugated to antigens. For example, when PorA was fused with the H. pylori HpaA antigen and expressed in Lactococcus lactis, PorA could significantly enhance the antibody response against the HpaA antigen after oral administration in mice [57].
3′5′-Cyclic di-adenosine monophosphate (c-di-AMP) is a bacterial second messenger that has strong mucosal adjuvant activity and numerous effects on the immune system, including type I IFN responses, promotion of Th1 and Th2 responses, increasing lymphocyte proliferation, and activation of APCs [58, 59]. Oral administration of recombinant L. lactis strains co-producing c-di-AMP and an anti-Trypanosoma cruzi antigen resulted in a T. cruzi-specific immune response
The Salmonella resistance to complement killing (RCK) protein plays an important role in interfering with complement killing and invading cells, including epithelial cells and APCs [60, 61]. The use of RCK as an oral adjuvant for the L. lactis vaccine strain successfully increased immune responses, conferring full protection against very-virulent infectious bursal disease virus (IBDV) challenge [62].
Protozoan-Derived Adjuvant Variant-Specific Surface Proteins
A novel oral adjuvant candidate could be achieved from parasitic protozoa, Giardia lamblia, which colonizes in the lumen of the upper small intestine of many vertebrate hosts. Serradell et al. [63] reported the variant-specific surface proteins (VSPs) from the Giardia lamblia surface can not only resist proteolytic digestion and extreme pH in GIT, as well as temperatures, but also stimulate host innate immune responses in a TLR-4-dependent manner. They constructed chimeric VSP-pseudotyped VLPs expressing hemagglutinin (HA) and neuraminidase (NA) of the influenza virus. These VSP-pseudotyped VLPs, but not plain VLPs, produced robust immune responses, protecting mice from influenza infection and HA-expressing tumors after oral immunization. This versatile oral vaccine adjuvant based on VSPs can be applied to antigens from different infectious agents or tumors and facilitate their use in remote areas where cold-chain for vaccine is not guaranteed.
Intestinal Immune Cells Targeting Peptide Adjuvants
Microfold cells (M cells), a unique subset of epithelial cells found in the epithelia covering MALT, such as Peyer’s patches, are used by the mucosal immune system to sample antigens in the GIT [64]. A variety of substances, including bacteria, viruses, and antigens, can be transported by M cells from the lumen to the underlying lymphoid tissues thanks to their great transcytotic ability [65–68]. Additionally, various antigens delivered by M cells can be sampled and captured by DCs positioned inside or beneath the epithelium [69, 70]. In addition, DCs can extend their probing dendrites into the lumen to sample commensal or microbial immunogens after passing through tight junctions to reach the gut epithelia [71]. These DCs subsequently migrate into the lymphoid follicles, where processed antigens are presented to B and T cells to sequentially trigger humoral (IgA) and T-cell immune responses [68, 72]. The aforementioned immunologic mechanisms of M cells and DCs can be exploited for the development of oral vaccine adjuvants. Therefore, targeting intestinal immune cells (such as M cells and/or DCs) is a promising strategy for developing oral vaccine adjuvants.
M Cell-Mediated Oral Adjuvants
In peroral mucosal vaccination, targeting M cells is considered a frontline prerequisite for effectively inducing antigen-specific immunostimulatory effects [73]. In the GIT, M cells are the antigen-collecting portals located on the FAE of Peyer’s patches and the gut-associated lymphoid tissue (GALT) of different species, which facilitate to transport antigens from gut lumen to the submucosal immune system [68, 74, 75]. M cells are believed to play a role in controlling gastrointestinal infection and immunity [73]. Therefore, M cell targeting might be a promising strategy for developing effective oral vaccine adjuvants [76].
M cell-targeting peptides Through phage display technology, Cho and colleagues [77] identified an M cell-homing peptide, CKS9 (CKSTHPLSC), which can facilitate the transcytosis of target antigen in M cells. In addition, according to Kim et al. [78], fusion of enhanced green fluorescence protein (EGFP) with another M cell-homing peptide, Col, could direct EGFP to bind to M cells and effectively transport it to mucosal immune induction sites to improve immune induction. Soon afterwards, with M cell-targeting peptides (Co1 or CKS9) as an oral vaccine adjuvant and LAB strain as an oral delivery vector, researchers tried to develop probiotic-derived oral vaccines against porcine diarrheal diseases, including porcine epidemic diarrhea (PED) [79] and swine dysentery [80], and obtained encouraging experimental outcomes after oral administration in mice.
In addition, the efficient uptake of antigens by M cells requires specific surface receptor molecules. Targeting the inherent receptors specifically expressed on the surface of M cells is another way to target M cells to deliver antigens to improve vaccine efficacy. Glycoprotein-2 (GP-2) is a glycosylphosphatidyl inositol anchoring protein that is specifically expressed on M cells and serves as a transcytotic receptor for luminal antigens [81]. Therefore, targeting GP-2 with specific ligands should increase antigen delivery to the immune initiation sites. Khan et al. [82] selected a GP2-binding peptide ligand, Gb-1, through phage library screening, which showed high binding affinity to GP-2. When fused with EGFP, Gb-1 significantly enhanced the uptake of EGFP by M cells compared with EGFP alone. Likewise, the Gb1-EGFP fusion induced effective mucosal and systemic immune responses after oral administration in mice. Therefore, exploiting the GP2-binding peptide Gb-1 for oral vaccine delivery would be a realistic approach.
Cytokine receptor activator of nuclear factor kappa B (NF-kB) ligand (RANKL). The proportion of M cells in intestinal epithelial cells is very low, accounting for approximately 1% of the total intestinal surface [68]. Therefore, if the number of M cells could be increased, it would be a promising technique to improve the effect of oral vaccines. It has been well-documented that the cytokine receptor activator of the nuclear factor Kappa B (NF-kB) ligand (RANKL) is a prevalent control factor for inducing M cells to differentiate from intestinal epithelial precursor cells by interacting with the cytokine receptor activator of NF-kB (RANK) expressed on the sub-epithelium of Peyer’s patches in the intestinal tract [83–85]. It has been proven that systemic administration of exogenous soluble RANKL (sRANKL) can correct the M-cell deficiency and uptake impairment in the Peyer's Patch [73]. In this regard, oral immunization by administering RANKL to induce the supraphysiological amount of M cells and then administering M cell-targeting antigens may be a viable approach to enhance the effect of oral vaccination.
A recombinant L. lactis IL-1403 producing and secreting soluble RANKL (sRANKL-LAB) constructed by Kim et al. could increase the expression of M cells in mice to be 1.51-fold higher than that in the untreated group through oral administration [83]. Maharjan et al. firstly administered intraperitoneally (or systemically) transmembrane RANKL (mRANKL) to mice and then delivered microparticulate antigen orally, which significantly increased the expression of M cells in FAE, showing similar effect as sRANKL-LAB [85]. They also demonstrated that RANKL-mediated transcytosis of antigens through M cells can enhance mucosal and humoral immunity. Choe et al. constructed RANKL-secreting L. lactis (LL RANKL) as an oral adjuvant for the aP2 subunit (soluble recombinant partial spike S1 protein from PEDV) vaccine loaded in hydroxypropyl methylcellulose phthalate (HPMCP) microspheres (HPMCP [aP2] plus LL RANKL) [86]. Their results showed that titers of virus-specific IgA antibodies in colostrum, and neutralizing antibodies in serum of sows vaccinated with HPMCP (aP2) plus LL RANKL increased significantly, and the survival rate of newborn suckling piglets delivered by sows vaccinated with HPMCP (aP2) plus LL RANKL was similar to that of piglets delivered by sows vaccinated with a commercial PED killed vaccine. These preclinical studies show that oral administration of RANKL is a promising adjuvant strategy, which could be used for effective oral vaccination and even oral therapeutic administration.
Dendritic Cell-Targeting Ligands
DCs represent the interface of the innate and adaptive immunity, and DCs play a pivotal role in priming T-cell immune responses against the inoculated antigen. Therefore, DCs are the major determinants of vaccination, so targeting oral vaccines to DCs is another strategy to enhance vaccination efficacy [87–89]. With DC-targeting peptides (DCpep, FYPSYHSTPQRP) as adjuvant, many researchers tried to utilize various LAB strains (including Lactobacillus plantarum, L. casei, Lactobacillus acidophilus, Lactobacillus saerimneri, L. lactis, etc.) as oral delivery vectors to develop oral vaccines for zoonotic or veterinary infectious diseases, such as Bacillus anthracis, and obtained good preclinical research results in animal model experiments of various diseases (Table 1). These research cases showed that modifying and specifically targeting a certain antigen to DCs can enhance antigen uptake.
Small Molecular Immunomodulatory Proteins
SMIPs are synthesized and secreted by a variety of tissue cells (mainly immune cells). They have many biological functions, such as regulating innate immunity and adaptive immunity, hematogenesis, cell growth, pluripotent stem cells and damaged tissue repair. To date, SMIPs used in the research of peroral vaccine adjuvants are mainly cytokines and Tα1.
Cytokine-Derived Oral Adjuvants
Cytokines are small proteins released by various cell types. Their functions are to stimulate, attract, and regulate the activity of immune cells (especially T cells), enhance the signal transduction of APCs, and sequentially improve the immune response to pathogens. They play a critical role in the regulation of innate and adaptive immunity [93, 94]. Cytokines have already been used orally to steer the immune system towards an increase in local cytotoxic T lymphocyte (CTL) activity and/or increased IgG and IgA titers. Some cytokines have been investigated as adjuvants for oral vaccines, and success has been reported in various preclinical studies, in which IL-2 is the most widely used oral adjuvant (Table 1). In particular, by genetically modifying probiotic strains (L. casei strain or Bacillus subtilis spores) to express corresponding host cytokines (such as IL-1β [95, 96], IL-2 [97–99], IL-6 [75], IL-12 [100], and granulocyte-macrophage colony-stimulating factor [GM-CSF] [101]), and oral coadministration with antigens or vaccine strains could significantly stimulate the production of specific antibody response in animals compared with the control groups. In some animal challenge tests, obvious protective immunity could be produced to fight against various infectious diseases, such as H. pylori infection [98, 99], Leishmania major infection [100], rabbit hemorrhagic disease (RHD) [97], and canine corona virus (CCV) [101]. Despite the above promising results, the potential safety concerns of cytokines need to be considered before using them as adjuvants [93]. According to the immunological properties of target antigens (or diseases), selecting specific and suitable cytokines as oral adjuvants needs to be based on the expected immune response of vaccination and its known influence on immune cells, but this is still one of the challenges of current immunological research. Overall, the optimal regimen of cytokines should be determined before starting clinical studies.
Thymosin α-1
Tα1 is a non-toxic immunomodified peptide hormone secreted by the thymus. It plays a very important role in cellular immune response by triggering T-cell maturation, augmenting T-cell function, developing antibody production, promoting reconstitution of immune defects, and increasing cytotoxic cells, Th1 and Th2 cytokine production, and IgG and intestinal sIgA production [102–104]. On account of its adjuvant attributes, by conjoining with the CSFV-E2 antigen and displaying it on the surface of L. plantarum, Tα1 could be used as an adjuvant of oral vaccine against classical swine fever virus (CSFV), which showed that Tα1 molecule adjuvant could enhance immune response and augment specific lymphocyte functions [102]. Therefore, Tα1 will be a promising adjuvant strategy in the development of an oral LAB vaccine [36].
Fc Region of Immunoglobulin G
As a potential adjuvant, the Fc region of IgG has attracted considerable attention. More and more evidence has demonstrated that fusion of the Ig Fc domain with the desired protein can facilitate dimerization of the protein, thus potently elevating the pharmacological and immunological characteristics of the protein [105–107], because the Fc region of IgG specifically binds to the FcRn (neonatal Fc receptor for IgG), which mediates IgG transport across the polarized epithelial cell lining on the mucosal surfaces [108]. As we know, IgG plays a predominant role in providing immune defense against foreign pathogens. Therefore, researchers have tried to target pathogenic antigens to FcRn as a new strategy to overcome intestinal epithelial barriers for mucosal vaccine delivery and drug therapy. Fc fusion proteins, or the recombinant proteins constructed by fusing the desired pathogenic antigens with the Ig Fc domain, have recently been utilized to produce vaccine candidates against infectious agents, including herpes simplex virus (HSV; gD-Fc) [109], pseudorabies virus (PRV) (gB-IgG2aFc) [110], HIV (Gag-Fc) [111], influenza A (H1N1) virus (3M2e-Fc) [112] and classical swine fever virus (CSFV) (E2-Fc) [113]. The aforementioned Fc fusion proteins could improve humoral and cellular immune responses by oral or intranasal immunization.
Biogenic Composite Oral Adjuvants
By combining two or more biogenic adjuvant materials to form a new composite adjuvant regimen, it is possible to improve mucosal immunity of target antigens in the intestine lumen. In this way, the advantages of each adjuvant could be fully utilized to enhance the overall immune effect. Until now, only the combination of intestinal immune cells targeting peptides and cytokines or the combination of two intestinal immune cells targeting peptides have been used as composite biogenic adjuvants in the development of oral vaccines, achieving good preclinical results. In particular, Li et al. [75] reported a novel biogenic composite mucosal adjuvant, IL-6-CKS9, which was a recombinant cytokine produced by conjugating an M cell-targeting peptide (CKS9) with the c-terminus of murine IL-6. Oral administration of recombinant L. lactis IL-1403 vaccine strain containing the above composite adjuvant promoted mucosal immune response. In addition, through combining the M cell-targeting peptide (Col) and DC-targeting peptide (DCpep) as a composite adjuvant, Ma et al. [114] genetically engineered a Lactobacillus vaccine strain that could target intestinal M cells and DCs and express COE antigen of PEDV. The recombinant strain efficiently induced anti-PEDV mucosal, humoral, and cellular immune responses in mice after oral administration. This suggests that the combination of Col and DCpep is a promising adjuvant strategy for oral probiotic vaccines. It is believed that more biogenic composite oral adjuvants will appear in the future.
Non-biogenic Oral Vaccine Adjuvants
Non-biogenic oral vaccine adjuvant materials are mostly polymeric microparticles/nanoparticles. They have many advantages, such as good biocompatibility, biodegradability, easy processing and modification, controllable surface properties, etc., and they could deliver and protect DNA and antigen protein of oral vaccines (or drugs) and control their release. Beyond that, they also possess mucosal absorptivity and immunostimulatory activity to activate or enhance immunity. Therefore, the application of non-biogenic adjuvant materials in oral vaccine research, and even in biomedical research, has become increasingly popular, showing great application prospects (Table 2).
Table 2.
Current developments in non-biogenic adjuvants for oral administration vaccines
| Adjuvant names | Explanations | Disease models (pathogens) | Vaccines (or candidate) formulations or antigens | Animal models | Results or immune responses in oral administration trials | References |
|---|---|---|---|---|---|---|
| Alum | ||||||
| Alum | Aluminium salts | HBV | Alum-adsorbed recombinant HBsAg from the commercial vaccine Engerix B® | BALB/c mice | Induced immune response at the protective level (≥10 mIU/mL) | [117] |
| PLG and PLGA | ||||||
| PLG | Poly(d,l-lactide-co-glycolide) | H. pylori infection | H. pylori-loaded PLG NPs | BALB/c mice |
Induced the H. pylori-specific mucosal and systemic responses Enhanced Th2-type responses |
[136] |
| PLG | As above | Rabies (CRV) | Encapsulated PLG+ CRV | Swiss albino mice |
Showed significantly higher anti-rabies virus IgG titer, virus-neutralizing antibody titers, and IgG2a and IgG1 titers The stimulation index of the lymphoproliferation assay was significantly higher The humoral, cellular immune response, and survival rates were significantly higher |
[138] |
| PLGA | Poly(d,l-lactic-co-glycolic acid) NPs | Aeromonas hydrophila | Recombinant OmpW of A. hydrophila encapsulated in PLGA NPs | Labeo rohita |
Protected against lethal challenge with A. hydrophila in rohu Inhibited A. hydrophila growth by sera from the high antigen group |
[205] |
| PLGA | As above | CMA | 18-Aaβ-lactoglobulin-derived peptides loaded PLGA NPs | C3H/HeOuJ mice |
Inhibited ex vivo whey-stimulated proinflammatory cytokine TNFα release Induced a dose-related partial prevention of CMA symptoms upon challenge to whole whey protein Silenced whey-specific systemic immune response |
[206] |
| Chitosan and its derivatives | ||||||
| Chitosan NPs | Prepared by ionotropic gelation | Photobacteriosis (Photobacterium damselae subsp. Piscicida) | DNA vaccine (pPDPimpdh) conjugated with CS-TPP NPs | Senegalese sole juveniles |
Significantly increased the concentration of lysozyme The non-specific immune responses and the specific humoral and cell-mediated immunity were observed |
[147] |
| Chitosan NPs | As above | Cystic echinococcosis | The multi-epitope vaccine (e.g. antigens combined with B cell, CTL and Th epitopes) encapsulated by chitosan NP | Mice | The concentration of multi-epitope antigen merged with microfold cells was high | [149] |
| Chitosan NPs | As above | Cow mastitis (E. coli) | Purified OmpA encapsulated with chitosan | Kunming mice |
Obtained the anti-serum titer (1:3200) Immune protection rate was 71.43% Downregulated the inflammation-related gene expression and the antioxidant factors Reduce injury in the liver and kidney |
[150] |
| Chitosan | As above | Salmonella serovar Enteritidis infection (S. enteritidis) | Salmonella subunit vaccine containing OMPs and flagellin protein loaded and flagellin protein surface-coated chitosan NPs | White leghorn layer chicks |
Increased TLRs, and Th1 and Th2 cytokine mRNA expression Enhanced specific systemic IgY and mucosal IgA antibody responses Reduced the challenge Salmonella load in the intestines |
[151] |
| Chitosan | As above | OVA-sensitized asthma | Chitosan-formulated OVA particles | OVA‐sensitized BALB/c mice |
Increases specific T-cell proliferation and IFNγ/IL-10 secretion Enhanced tolerance induction in mice with asthma Dramatically reduced AHR, lung inflammation, eosinophil numbers Induced antigen-specific Th2 responses |
[207] |
| Chitosan | As above | Enterovirus 71 | Recombinant enterovirus 71 VP1 formulated with chitosan | ICR mice |
Induced VP1-specific IgA antibodies and serum-specific IgG and neutralization antibodies Induced high levels of Th1-, Th2- and Th3-type immune responses Conferred survival rate up to 30% |
[208] |
| Chitosan NPs | As above | Edwardsiella tarda infection | Recombinant outer membrane protein A of Edwardsiella tarda encapsulated in chitosan NPs | Labeo fimbriatus |
Produced higher antibody levels Had superior protection over the inactivated whole cell E. tarda vaccine Conferred improved protection against E. tarda mortality |
[209] |
| N-trimethyl chitosan (TMC) | Prepared by ionic complexation with pentasodium TPP | Brucellosis (Brucella melitensis) | Recombinant B. melitensis Omp31 loaded onto TMC NPs | BALB/c mice |
Increased vaccine residence time in the intestine Enhanced vaccine permeation and immunogenicity Stimulated maturation of DCs Induced specific IgG2a production, high levels of IFNγ, IL-12, IL-17 and Th1–Th17 production Increased IgA levels Significantly protected against B. melitensis 16M |
[153] |
| Mannosylated chitosan nanoparticles (MCS NPs) | Prepared by the ionic gelation method with TPP | NM | BSA-loaded Eudragit® L100-coated MCS NPs | Sprague Dawley rats and BALB/c mice |
MCS NPs were accumulated more specifically into PPs Elicited strong systemic IgG antibody and mucosal IgA responses |
[155] |
| UEA-1 | ||||||
| UEA-1 | Ulex europaeus agglutinin 1 | Hepatitis B | HBsAG encapsulated liposomes conjugated with UEA-1 | BALB/c mice |
Enhanced binding to M cells Induced higher sIgA and cytokine levels |
[161] |
| Alpha-Galactosylceramide (α-GalCer) | ||||||
| α-GalCer | Synthetic iNKT-cell agonists | AIDS (HIV) | Combination of the CTL-inducing HIV envelope peptide (R15K peptide) and the synthetic glycolipid α-GalCer | BALB/c mice |
Induced efficient and broader systemic and mucosal antigen-specific immune responses Led to immune recognition of the cognate HIV envelope protein Repeated dosing of α-GalCer does not adversely affect the peptide-specific CTL responses |
[162] |
| α-GalCer | As above | Diarrheal infections (ETEC and cholera) | SmPill® vaccine formulation that combines α-GalCer coating whole cell killed E. coli overexpressing JT-49 | BALB/c mice | Promoted CFA/I-specific IgA responses in the intestinal mucosa in addition to serum IgG | [164] |
| α-GalCer | As above | Severe diarrheal disease (V. cholerae) | SmPill® minispheres contained formalin-killed V. cholerae Hikojima MS1242 bacteria and α-GalCer | C57BL/6 and BALB/c mice | Significantly enhanced intestinal and serum antigen-specific antibody responses | [14] |
| α-GalCer | As above | Chronic gastric infection (H. pylori) | Intragastric immunization with a whole-cell killed H. pylori antigen candidate vaccine with α-GalCer |
C57BL/6, IL-17RA−/− and IL-1RI−/− mice |
Induced effective immune protection against H. pylori infection with similar magnitude as cholera toxin as adjuvant Enhanced intestinal antigen-specific IgA responses to a whole-cell killed H. pylori antigen |
[165] |
| Synthetic TLR-agonist adjuvants | ||||||
| GS-986 | TLR7 agonist | SIV | Ad26/MVA vaccination and GS-986 administration | Rhesus monkeys |
Improved virologic control and delayed viral rebound following ART discontinuation Led to innate immune stimulation and cellular immune activation |
[168] |
| CpG-ISS (or CpG ODN 1018) | TLR9 agonist | NV | Oral co-delivery of NV VLPs with CpG-ISS | BALB/c mice | Augmented local VLP-specific fecal IgA titers | [175] |
| CpG ODN 1826 | TLR9 agonist | OVA-sensitized asthma | OVA in combination with CpG-ODN | BALB/c mice | Induced OVA-specific T-cell proliferative response, IgG, and IgA | [171] |
| Multiple non-biogenic-composite (or multiple microparticles/nanoparticles) adjuvants | ||||||
| Mannosylated PNPs | Mannosamine-coated poly(anhydride) NPs | OVA-sensitized asthma | OVA-loaded mannosylated polyanhydride NPs | BALB/c mice |
Elicited higher and balanced systemic-specific antibody responses Elicited higher level of intestinal sIgA compared with SC administration Strong, long-lasting systemic and mucosal immune responses |
[179] |
| UEA-MPL/lipid NPs | UEA-1 conjugated PLGA-lipid nanoparticles containing a TLR-agonist monophosphoryl lipid A | OVA-sensitized asthma | OVA-UEA-MPL/lipid NPs | BALB/c mice |
Almost exclusively adhered to M cells Led to specific absorption and continuous retention in the PP Effectively transported by M cells and captured by mucosal DCs Stimulated effective mucosal IgA and serum IgG antibodies |
[160] |
| UEA-1 LACNP | UEA-1 lectin-anchored alginate-coated chitosan NPs | NM | UEA-1 LACNP-BSA | BALB/c mice | Induces efficient systemic and mucosal immune responses against BSA | [210] |
| MPLA/PLGA | Immunostimulant MPL incorporated in PLGA NPs | OVA-sensitized asthma | OVA and the MPLA incorporated in PLGA | BALB/c mice |
Induced a stronger IgG immune response than the control formulations Generated significantly higher IgA titers |
[178] |
| LTA-PLGA NPs | Lectin-anchored PLGA NPs | Hepatitis B | LTA-grafted PLGA nanoparticles encapsulating hepatitis B surface antigen | BALB/c mice |
Shown fourfold increase in the degree of interaction with the BSM Elicited strong mucosal and systemic response |
[180] |
| HP55/PLGA NPs | Acid- resistant HP55/PLGA NPs | H. pylori infection | H. pylori recombinant antigen CCF encapsulated acid-resistant HP55/PLGA NPs | BALB/c mice |
Induced high levels of urease-specific antibodies and memory T-cell responses 43% of mice were completely protected after H. pylori challenge |
[142] |
| Chitosan-alginate capsules | NM | KHV disease | Probiotic vaccine (pYG-KHV-ORF81/LR CIQ249 expressing KHV ORF81 protein) encapsulated by chitosan-alginate capsules | Koi carp |
Effectively induced antigen-specific IgM Displayed effective KHV-neutralizing activity Provided 85% protection rate for koi carp against KHV challenge |
[148] |
| Chitosan/alginate microparticles | NM | Fowl typhoid (S. gallinarum) | Live 9R vaccine coated with chitosan/alginate microparticles | Chicks |
Upregulated IFNγ expression 100% protection No significant difference between oral and subcutaneous administrations Prevented vaccine destruction in the GIT |
[211] |
| Alginate-coated chitosan NPs | NM | Hepatitis B | Recombinant hepatitis B vaccine with alginate-coated chitosan NPs | Mice | Have potential use as a delivery system for oral vaccination with recombinant HBsAg | [182] |
| CpG ODN-loaded alginate coated chitosan NPs | NM | Schistosomiasis (Schistosoma mansoni) | SmRho-CpG ODN-loaded alginate-coated chitosan NPs | C57BL6 mice |
Showed significant modulation of granuloma reaction Presented significant levels of protection against infection challenge with S. mansoni worms |
[181] |
| CpG ODN-loaded PLGA NPs | NM | Campylobacteriosis (C. jejuni) | Combination of PLGA-encapsulated CpG and C. jejuni lysate | Commercial broiler chicks |
Reduced bacterial counts in cecal contents by 2.42 log10 Anti-C. jejuni IgG antibody titers were significantly higher |
[183] |
| ALG-CHT-LDH NPs | Alginate-chitosan coated layered double hydroxide nanocomposites | NM | ALG-CHT-LDH@BSA | NM | Significantly enhance the attachment and internalization of proteins in the Caco-2 cells and macrophages | [157] |
| PLG/PLA microsphere | Poly-(d,l-lactide-co-glycolide) and poly-(l-lactic acid) | Seven common respiratory pathogens | LW 50020 encapsulated into PLG and PLA microsphere | BALB/c mice |
Enhanced immune response Immunomodulation was statistically significant compared with free LW 50020 |
[137] |
| CS/PLGA-NPs | Chitosan-coated poly (lactic-co-glycolic) acid NPs | NDV | pFDNA-CS/PLGA-NPs | Chickens | Induced stronger cellular, humoral, and mucosal immune responses | [212] |
The immune response results of the above examples are all the results of the oral immunization test
AHR airway hyperresponsiveness, AIDs acquired immune deficiency syndrome, ART antiretroviral therapy, BSA bovine serum albumin, BSM bovine submaxillary mucin, CMA cow’s milk allergy, CpG-ODN CpG oligodeoxynucleotides, CRV concentrated rabies virus, CTL cytotoxic T lymphocyte, DCs DC dendritic cells, ETEC enterotoxigenic Escherichia coli, GIT gastrointestinal tract, HBsAg hepatitis B surface antigen, HBV hepatitis B virus, HIV human immunodeficiency virus, IFN interferon, Ig immunoglobulin, IL interleukin, iNKT invariant natural killer T cell, KHV Koi herpes virus, MPLA monophosphoryl lipid A, MVA modified vaccinia virus Ankara, NDV Newcastle disease virus, NM not mentioned, NPs nanoparticles, NV Norwalk virus, OMPs outer membrane proteins, OmpA outer membrane protein A, OmpW outer membrane protein W, OVA ovalbumin, PNPs polyanhydride nanoparticles, SC subcutaneous administration, sIGA secretory immunoglobulin A, SIV simian immunodeficiency virus, Th T helper, TLRs toll-like receptors, TNF tumor necrosis factor, TPP tripolyphosphate, VLPs virus-like particles
Alum
Alum, also referred to as ‘aluminium salts’, encompass aluminium potassium sulphate, aluminium hydroxide, aluminium phosphate, and amorphous aluminium hydroxyphosphate sulfate [115]. Alum is one of the most widely accepted vaccine adjuvants and is a component of several licensed parenteral vaccines [116]. Kapusta et al. [117] reported oral administration with nanogram doses of alum-adjuvanted hepatitis B surface antigen (HBsAg) in mice-induced humoral immune response at the protective level. However, alum is unable to enhance cell-mediated Th1 or CTL responses, which are vital to control most intracellular pathogens [118]. Furthermore, alum is considered a poor inducer of mucosal immunity [37].
Polymer-Based Microparticle/Nanoparticle Oral Adjuvants
To overcome the harsh environment of the GIT, different types of polymer-based nanoparticles (including synthetic and natural polymers) have been widely studied for the preparation of various microparticle/nanoparticle vaccines (or nanoparticle adjuvants) for the GIT due to their biocompatibility, biodegradability, non-toxic nature, and ease of modification into desired shapes and sizes, as well as protecting the vaccine bioactivity from adverse situations [2, 119]. Polymer-based nanoparticle adjuvants are made of polymers such polyanhydride, poly (ethylene-glycol), PLG, PLGA, poly(lactic acid) [PLA], chitosan, alginate, and their derivatives, among others, and they have demonstrated enhancement of intestinal immune responses in vaccines for preventing various infections and treating various inflammatory diseases [7]. In this part, the application and research progress of polymer-based microparticles/nanoparticles as adjuvants for the peroral vaccines were reviewed.
Polyanhydride-Based Oral Adjuvant Materials
Polyanhydrides (PAHs), a class of synthetic biodegradable, non-cytotoxic, biocompatible polymers, are polymerized by methyl vinyl ether and maleic anhydride [120, 121]. PAHs are inherently highly reactive to water, thus leading to relatively rapid hydrolytic degradation, breaking down into carboxylic acids without cytotoxicity [121]. PAHs have been used in vaccine delivery systems for a long time, and polyanhydride nanoparticles (PNPs) are licensed for oral drug delivery in the UK [121–123]. In fact, PAHs are also a promising oral vaccine encapsulating material with the function of adjuvant and carrier. First, polyanhydride particles are cleaved in the gut to expose carboxylic acid groups that form hydrogen bonds with the hydroxyl groups of glycoproteins in the gut mucus, giving polyanhydride particles their mucoadhesive properties [124, 125]. Second, it has been reported that polyanhydride particles possess intrinsic adjuvant properties, which can activate APCs and regulate the immune responses [121, 126]. Furthermore, polyanhydride particles have been demonstrated to be able to provide sustained release of protein antigens via surface erosion [121, 125]. In addition, polyanhydride materials can be made into nano-encapsulated formulations by nanotechnology, which can exert better adjuvant effects. PNP-based vaccines have been shown to successfully encapsulate and release antigens, activate B and T cells, and induce both antibody- and cell-mediated immunity towards a variety of immunogens [127]. Moreover, PNPs act as agonists of various TLRs (TLR2, 4, and 5) [10, 126], innate immunity, complement system, and APCs to modulate the immune responses and induce long-lasting immunity [121, 126, 128]. Renu et al. reported that mucoadhesive PNPs could protect the vaccine cargo and deliver it to intestinal immune sites to elicit robust mucosal immunity and mitigate Salmonella colonization and shedding [125]. Overall, PNPs have potent immune adjuvant properties when administered orally and can target immune cells of chickens [125], mice [129, 130], rats [131, 132], and other animals.
Poly(d,l-Lactide-co-Glycolide) and Poly(d,l-Lactic-co-Glycolic Acid)
PLG is a biodegradable and biocompatible polymer [133]. Microparticles prepared from PLG have been proven to be effective adjuvants for a variety of antigens because microencapsulation of PLG can protect antigens from adverse degradation, allow sustained and prolonged release of antigens for a long time, and enhance uptake of antigen by APCs [134]. These APCs containing PLG-microparticles are then delivered to specific lymphoid compartments, such as the spleen and mesenteric lymph nodes, where they effectively present antigenic epitopes to T lymphocytes, especially Th1 and Tc, thus inducing strong specific cell-mediated immunity (Fig. 1e1) [134, 135], which is urgently needed for eliminating intracellular pathogens in host cells. Kim et al. reported that using H. pylori lysates encapsulated in PLG nanoparticles as an oral vaccine candidate could induce the H. pylori-specific mucosal and systemic responses in mice, and enhanced Th2-type responses [136]. Kofler et al. reported that the pulmonary and serum immune responses of BALB/c mice were enhanced by oral immunization with LW50020 encapsulated with PLG microspheres [137]. Ramya et al. used PLG microspheres as an oral delivery system for β-propiolactone inactivated concentrated rabies virus (CRV) and found that Th1-mediated cellular immunity was activated after oral administration of PLG+CRV in mice [138]. In addition, PLG microspheres also have many potential advantages in gene therapy [133].
PLGA nanoparticles are US FDA-approved biocompatible and biodegradable polymers, which are widely used in preclinical vaccine delivery. PLGA has the functions of delivery device, protection, sustained release of encapsulated antigen, and enhancement of antigen uptake during vaccination [139–141]. In addition, PLGA combined with pH-responsive materials can adapt to the extreme GIT more efficiently and has the potential to become an oral vaccine adjuvant. Tan et al. designed an acid-resistant PLGA nanoparticle (HP55/PLGA-CCF) using pH-responsive material, HP-55, which was an effective immunomodulator and an oral carrier to enhance the efficacy of subunit vaccines. Mice immunized with HP55/PLGA-CCF nanoparticles could induce high levels of urease-specific antibodies and memory T-cell responses [142]. As pointed out by Munang’andu and Evensen [143], adjuvants that serve as antigen delivery vehicles and immunostimulants are able to enhance antigen uptake by APC. Furthermore, PLGA has the above two inherent adjuvant properties [144]. PLGA NP-rOmpW (i.e., the outer membrane protein W [OmpW] of Aeromonas hydrophila encapsulated in PLGA nanoparticles) provided dose-dependent protection against A. hydrophila infection in Rohu (Labeo rohita Hamilton) after oral administration [140]. In general, the design of PLGA nanoparticles as an oral immune adjuvant is a promising strategy to improve antigen uptake and vaccine efficiency.
Chitin, Chitosan and Their Derivatives
Chitin particles possess TLR-2-dependent adjuvant activity and can augment the Th1, Th2, and Th17 antigen-specific immune responses when admixed with protein antigens [145]. Chitosan (CS), a deacetylated form of chitin, is a polysaccharide composed of N-acetyl-d-glucosamine and d-glucosamine [146]. Because of its low toxicity, excellent biocompatibility, biodegradability, antimicrobial activity, mucoadhesive properties, and permeation-enhancing effects, chitosan has been widely used as a potential excipient for the oral delivery of DNA, peptides, and live attenuated virus [147–151]; however, its limited mucoadhesive strength and low water solubility at neutral and basic pHs are considered as two major drawbacks of its biomedical applications. The chemical modification of chitosan results in quaternized chitosan [152] or its derivatives, such as N-trimethyl chitosan (TMC) [153], O-2′-hydroxypropyltrimethyl ammonium chloride chitosan (O-2′-HACC) [154], and mannosylated chitosan (MCS) nanoparticles [155]. This enhanced the mucoadhesive properties of chitosan. In addition, many researchers are trying to optimize chitosan nanoparticles and combine them with other nano-materials for composite adjuvants, which can promote a more efficient immune function and serve as a promising carrier for oral protein vaccine delivery [146].
Alginate and Its Derivatives
Alginate is a non-toxic, biodegradable, low cost, readily available polysaccharide copolymer containing (1-4)-linked β-d-mannuronate and α-l-guluronate residues, and is a mucoadhesive, biocompatible, non-immunogenic substance [2]. Alginate has been widely used in drug delivery because of its ability to contract in the stomach and release its cargo in the intestine. Alginate polymer as a single component is rarely used as an adjuvant. Alginate would usually be anchored/coated with chitosan or other electropositive materials by chemical modification to develop alginate-based composite adjuvant formulations for oral protein antigens (or vaccines) delivery (Tables 2, 3), such as alginate-coated chitosan microparticles (ACMs) [156] and alginate-chitosan coated layered double hydroxide nanoparticles (LDHs) nanocomposites (ALG-CHT-LDH) [157].
Table 3.
Current developments in biogenic and non-biogenic combined composite adjuvants for oral administration vaccines
| Adjuvant names | Explanations | Disease models (pathogens) | Selected antigens or vaccines | Animal models or cell models | Results or immune responses | References |
|---|---|---|---|---|---|---|
| PLGA-IL-2, PLGA-IL-18, PLGA-GM-CSF | PLGA NPs combined with plasmid encoding IL-2, IL-18, or GM-CSF | FMD | PLGA-VP013/IL-2, PLGA-VP013/IL-18, PLGA-VP013/GM-CSF | Guinea pigs |
Elicited significantly higher FMDV-specific antibody levels Significantly increased neutralizing antibodies Dramatically enhanced cellular immunity |
[186] |
| CKS9-CNs | CKS9-immobilized chitosan NPs | None | None | In vitro transcytosis assay and closed ileal loop assay | Transported more effectively across the M cell model and accumulated more specifically into PP regions | [77] |
| Flagellin-PNPs | Polyanhydride nanoparticles coated with Salmonella enteritidis-derived flagellin | OVA-sensitized asthma | OVA-loaded flagellin nanoparticles | BALB/c mice |
Elicited higher and balanced systemic specific antibody responses Elicited higher level of intestinal sIgA compared with SC administration Strong, long-lasting systemic and mucosal immune responses |
[179] |
| F-PNPs | S. enteritidis flagellar protein-coated polyanhydride NPs | Salmonellosis (S. enteritidis) | Salmonella OMPs and flagellar protein-entrapped and surface flagellar protein-coated PNPs | Austra White laying chicks |
Induced higher OMP-specific IgG response and secretion of Th1 cytokine IFN-γ Enhanced CD8+/CD4+ cell ratio Increased OMP-specific lymphocyte proliferation Upregulated the expression of TLR2 and 4, TGF-β, and IL-4 cytokine genes Cleared Salmonella cecal colonization in 33% of vaccinated birds |
[125] |
| CS NPs-F | Flagellin protein-coated CS NPs | Salmonellosis (S. enteritidis) | OMPs-F-CS NPs | Chicks |
The particles were localized in ileal Peyer’s patches Induced significantly higher OMP-specific mucosal IgA and lymphocyte proliferation response Increased the expression of TLR2, TLR4, IFN-γ, TGFß and IL-4 mRNA expression |
[213] |
| CKS9-WSC-PLGA MPs |
Porous PLGA MPs coated with M cell-homing peptide (CKS9)-coupled water-soluble chitosan |
Swine dysentery (B. hyodysenteriae) | Membrane protein B of B. hyodysenteriae loaded into porous PLGA MPs coated with the WSC conjugated with CKS9 | BALB/c mice |
Enhanced M cell targeting and transcytosis ability Showed elevated secretory IgA responses and systemic IgG responses Induced both Th1- and Th2-type responses |
[185] |
| AlgChiPs | Alginate-coated chitosan particles | HBV | Recombinant HBsAg encapsulated into AlgChiPs | C57BL/6 mice | Induced serum anti-HBsAg IgG and anti-HBsAg sIgA | [214] |
The immune response results of the above examples are all the results of the oral immunization test
CS chitosan, FMD foot and mouth disease, GM-CSF granulocyte-macrophage colony-stimulating factor, HBsAg hepatitis B surface antigen, HBV hepatitis B virus, IFN interferon, Ig immunoglobulin, IL interleukin, MPs microparticles, NPs nanoparticles, OMPs outer membrane proteins, OVA ovalbumin, PLGA poly(d,l-lactic-co-glycolic acid), SC subcutaneous, sIGA secretory immunoglobulin A, TGF transforming growth factor, Th T helper, TLR toll-like receptor
M Cell-Targeting Polymeric Particles (Ulex Europaeus Agglutinin-1)
UEA-1 is a lectin with specific binding activity to epitopes containing α-L-fucose [158]. UEA-1 can exclusively bind to M cells of mouse small intestine [159] and has been identified as an M cell-selective molecular marker [160]. Bioactive UEA-1 has been explored in the present investigation for targeted oral immunization. Gupta and Vyas reported UEA-1 conjugated liposomes as an oral M cell-targeted vaccine delivery vector [161]. In their study, the UEA-1 conjugated liposomes were predominantly targeted to the M cells. The serum anti-HBsAg IgG titer was obtained after oral immunization with HBsAg-encapsulated liposomes conjugated with UEA-1 for 3 consecutive days. The boosting immune effect was comparable with the titer recorded after single intramuscular immunization with alum-HBsAg [161]. Moreover, UEA-1-conjugated liposomes induced higher sIgA levels in mucosal secretions and cytokine levels in the spleen homogenates [161].
Alpha-Galactosylceramide
α-GalCer, a synthetic glycolipid, is a potent inducer of the invariant natural killer T (iNKT) cells, which are an important innate immune cell type [162]. α-GalCer can be presented by the CD1d molecules on the APC to NKT cells [163], which leads to activation and expansion of NKT cells, and subsequently induces full maturation of DCs in the spleen after immunization [162]. Therefore, α-GalCer is identified as a non-toxic oral adjuvant. It has recently been shown that α-GalCer acted as an oral active adjuvant to induce T-cell immunity against pathogenic bacteria and viruses through efficient activation/maturation of DCs. According to studies, α-GalCer potentiated mucosal immune responses to the HIV model envelope peptide (R15K peptide) [162], ETEC vaccine [164], V. cholerae vaccine [14], and whole-cell killed (WCK) H. pylori candidate vaccine [165] through oral immunization. The study by Davitt et al. demonstrated that α-GalCer was as effective as the ‘gold standard’ mucosal adjuvant CT in promoting intestinal IgA responses against a novel ETEC antigen [164]. In another study by Davitt et al., the addition of α-GalCer enhanced mucosal immunogenicity of Dukoral®, the most widely licensed oral cholera vaccine (OCV) internationally, and significantly increased intestinal anti-LPS and anti-cholera toxin B subunit (CTB) IgA responses against V. cholerae infections [14]. Longet et al. demonstrated that oral immunization of H. pylori WC antigen adjuvanted with α-GalCer significantly reduced bacterial loads in the stomach of H. pylori-infected mice; this reduction was IFNγ- and CD1d-dependent, similar to CT as adjuvant [165]. In conclusion, α-GalCer is an effective mucosal adjuvant for oral immunization and can enhance the mucosal responses of IgA and Th1 in mice, but its safety and efficacy in humans still warrant further evaluation. In addition to its impressive oral adjuvant effects in mice, α-GalCer has been tested in clinical trials for the treatment of cancer and hepatitis, in which its safety has been assessed [14].
Synthetic Toll-Like Receptor (TLR)-Agonist Molecules
As mentioned earlier, TLR molecules have been the target of many new mucosal vaccine candidates. Targeting one or more TLR(s) might activate sensors of innate TLR pathogens and promote intracellular signaling cascades that lead to upregulation of the production of chemokines and cytokines required for DC maturation, which results in increased magnitude and quality of immune responses [93, 166]. Some synthetic TLR ligands could also activate TLR signals and subsequently promote immune responses, which have been exploited as potential adjuvants of mucosal vaccines. For example, incorporation of the TLR4 agonist monophosphoryl lipid A (MPL) into nanoparticle vaccines could contribute to triggering TLR signaling with mucosal DCs and subsequently improve the capture efficiency of vaccines [160]. The TLR 7/8 agonists R848 have showed great potential as oral vaccine adjuvants because they can directly activate APCs and enhance both humoral and cellular immune responses, especially Th1 responses [167]. According to Borducchi et al., oral administration of Ad26/MVA combined with the TLR7 agonist GS-986 could decrease the level of SIV viral DNA in lymph nodes and peripheral blood, as well as control and delay virologic rebound following antiretroviral therapy discontinuation in SIV-infected Rhesus Monkeys [168].
In addition, CpG oligodeoxynucleotides (CpG-ODN) are another promising synthetic TLR-agonist adjuvant. They are short single-stranded synthetic DNA molecules that can activate the immune system and have been found to be effective in the prevention and treatment of infectious diseases, allergies, and cancers [16, 169]. CpG-ODN, a ligand of TLR9, can activate TLR9 on B-lymphocytes and DCs, showing potent activity in stimulating antigen presentation and inducing antigen-specific immune response towards the Th1 phenotype [170]. Alignani et al. reported CpG-ODN-loaded ovalbumin (OVA) induced specific mucosal and systemic immune response in mice after oral administration [171]. CpG-ODN has different classes, such as CpG-ODN 2007 [172], CpG-ODN 1668 [173], and CpG-ODN 1826 [174], and has also shown potent mucosal adjuvant activity. Hjelm et al. [175] used a panel of TLR agonists (PIC [TLR3], FLAG [TLR5], GARD [TLR7], CpG [TLR9], CpG-ISS [CpG 1018, alternate CpG motif, TLR9], and CL097 [TLR7/8]) as adjuvants combined with Norwalk VLPs (NV VLPs) coadministered to mice through intranasal and oral routes to determine the mucosal adjuvant activity of these immunomodulators. Of these, intranasal co-delivery of VLPs with TLR7 or TLR9 agonists (i.e., GARD or CpG) produced the most robust and broad-spectrum immune response, but oral administration with other TLR agonists (i.e., PIC, FLAG, and CL097) could not consistently enhance VLP-specific immune responses in mice.
According to our knowledge, there are no human trials using TLR agonists as oral vaccine adjuvants. These above studies are preclinical studies, indicating that TLR plays an important role in inducing immune response in the oral route.
Composite Non-biogenic Material Adjuvants
Through chemical modification or nanotechnology, the physicochemical properties of non-biogenic adjuvant materials (or polymeric nanoparticles) can be improved by combining them with another non-biogenic adjuvant material. Their advantages can be complementary, which is beneficial to enhance the interaction between nanoparticle adjuvant and intestinal endocytosis pathways [176, 177]. For instance, UEA-1 is the M-cell selective molecular signature, which could exclusively adhere to M cells, and MPL is a TLR agonist. With a combination of UEA-1 and MPL, Ma et al. [160] reported that the composite material, UEA-MPL-conjugated PLGA-lipid nanoparticles, can be effectively transported by M cells and captured by mucosal DCs, showing the potential of an attractive oral vaccine delivery system for boosting oral immunity. Ma et al. found that OVA-UEA-MPL/lipid nanoparticles stimulated the most effective mucosal IgA and serum IgG antibodies during oral vaccination [160]. Sarti et al. used MPL-conjugated PLGA nanoparticles as an oral adjuvant of OVA in mice [178], and compared with the control formulation group, it generated significantly higher IgA titers, which indicated that MPL-PLGA nanoparticles had the ability to induce mucosal immunity. Salman et al. reported that mannosamine-coated poly(anhydride) nanoparticles as an oral composite adjuvant of OVA induced strong, long-lasting systemic and mucosal immune responses than the non-conjugated vectors [179]. Moreover, Mishra et al. demonstrated that LTA (Lotus tetragonolobus from Winged or Asparagus pea)-anchored PLGA nanoparticles could elicit strong mucosal and systemic response and hence could be a promising M cell-targeting adjuvant for oral mucosal immunization against hepatitis B [180]. Alginate-coated chitosan nanoparticles are of interest because of their great stability and immunostimulatory properties. They can effectively transport antigens into the M cells and subsequently induce significant immune responses in serum IgG and mucosal sIgA levels [156, 181]. Borges et al. demonstrated that alginate-coated chitosan nanoparticles showed potential as a delivery system for oral recombinant HBsAg [182]. Most recently, Yu et al. demonstrated that alginate-chitosan coated layered double hydroxide nanocomposites (ALG-CHT-LDHs) showed great potential in oral protein vaccine delivery [157]. In addition, Taha-Abdelaziz et al. reported that oral administration of PLGA-encapsulated CpG ODN, and Campylobacter jejuni lysate reduced cecal colonization by C. jejuni in chickens [183].
The above studies show that the physicochemical properties of single non-biogenic adjuvant material can be improved by comprehensive combination of double or triple, or even quadruple, adjuvant materials through nanotechnology, so as to correctly match the size, electric charge, hydrophobicity, and other physicochemical properties of antigen, and so that antigen can cross the mucosal barriers and target APCs [184]. Through the appropriate combination of a variety of non-biogenic adjuvant materials, its advantages could be developed and its disadvantages could be avoided, so as to construct a universal and powerful oral vaccine carrier or adjuvant.
Biogenic and Non-biogenic Combined Composite Material for Oral Adjuvants
Nowadays, using conjugation techniques to combine biogenic adjuvants (such as M cell-targeting peptides, bacterial flagellin, or cytokines, etc.) with other non-biogenic adjuvants to create composite adjuvants can give full play to the advantages of the activity of each adjuvant, thus enhancing the overall activity of adjuvants (Table 3). The composite adjuvant, PLGA microparticles coated with chitosan-coupled M cell-homing peptide (CKS9), can strengthen the targeting ability to M cells, and the mucosal and systemic immune responses were induced when it was used to deliver swine dysentery vaccine [185]. As mentioned previously, PNPs are natural mucoadhesive polymers that could efficiently deliver antigens to the GALT [179], and flagellar protein possesses potent immune adjuvant activity. Renu et al. designed a Salmonella subunit vaccine (OMPs-F-PNPs) that consisted of PNPs containing immunogenic Salmonella OMPs and entrapped flagellar (F) protein and surface F-protein-coated PNPs. The vaccine could induce specific immune response to mitigate Salmonella colonization in the intestines of chickens vaccinated orally [125]. Yang et al. used PLGA nanoparticles combined with cytokine as adjuvant to deliver DNA vaccine of foot and mouth disease, which significantly enhanced its immunogenicity than naked DNA [186]. Generally, the conjugation of biogenic adjuvant and non-biogenic adjuvant as composite adjuvants is another frequently used and promising way to assist the delivery of oral vaccine and enhance its immunogenicity.
Concluding Remarks, Challenges, and Future Perspectives
The complex and harsh environment in GIT leads to the weak immunogenicity of peroral mucosal vaccines. Preparation of effective adjuvants to enhance the immune response is an integral part of the development of oral vaccines. Over the past few decades, several biogenic and/or non-biogenic adjuvants have been used in the trials of various peroral mucosal vaccines to enhance their immune responses. At present, among the aforementioned adjuvant candidates of peroral vaccines, only dmLT has undergone human clinical trials and further passed clinical phase I and II trials [187, 188], while other adjuvant candidates are still in animal (or veterinary or aquatic) experimental stage (Fig. 2). In this review, some adjuvants have been tested on farm animals, such as pigs, birds, fish, etc., to develop veterinary vaccines (or adjuvants) (Fig. 2). For the development of veterinary vaccines (or adjuvants), the target animals are the most ideal animal models. Mice (especially BALB/c mice) are commonly used animal models for preclinical trials of adjuvants (or vaccines) in animals or humans (Fig. 2). Animal models play a critical role in the in vivo study of the immunology and pharmacology of oral adjuvant (or vaccines) candidates, as well as the evaluation of potential applications in humans. However, it is undeniable that many animal models used in oral adjuvant (or vaccines) tests have limited predictive value for the human response to oral adjuvants (or vaccines) in terms of both efficacy and toxicology [189]. Among the examples of oral adjuvants (or candidates) we summarized (Tables 1, 2, 3), there were few reports focusing on the purposeful selection of animal models to evaluate the efficacy of oral adjuvants. Many researchers chose animal models for their animal experiments based on the disease types they were studying, instead of the oral adjuvants, which made it difficult to translate the positive effects of oral adjuvants on animal models to humans and required considerable analysis and debate. It is necessary to improve the existing animal models to make them more predictive for humans.
Fig. 2.
List of oral adjuvant candidates developed and their corresponding in vivo tests. According to the physicochemical properties, oral adjuvants could be divided into biogenic, non-biogenic, and a biogenic and non-biogenic combined composite. In vivo tests for oral adjuvant development have involved humans, rabbits, fish, rodents, pigs, primates, canines, and chickens. No connection means the in vivo test has not yet been carried out. α-GalCer alpha-Galactosylceramide, c-di-AMP 3′5′-cyclic di-adenosine monophosphate, CKS9 M cell-targeting peptide, Co1 M cell-specific peptide ligands, CpG-ODN CpG oligodeoxynucleotides, DCpep dendritic cell-targeting peptide, dmLT double-mutant heat-labile toxin, FnBPA fibronectin binding protein A, GM-CSF granulocyte-macrophage colony-stimulating factor, MCS NPs mannosylated chitosan nanoparticles, MDP muramyl dipeptide, mmCT multiple mutant cholera toxin, MPL monophosphoryl lipid A, O-2′-HACC O-2′-hydroxypropyltrimethyl ammonium chloride chitosan, PLG poly(d,l-lactide-co-glycolide), PLGA poly(d,l-lactic-co-glycolic acid), RANKL receptor activator of NF-kB ligand, RCK Salmonella resistance to complement killing, SMIPs small molecular immunomodulatory proteins, TMC trimethyl chitosan, UEA-1 ulex europaeus agglutinin-1
An obvious advantage of biogenic adjuvants is that they can be constructed or optimized by genetic engineering. On the one hand, gene editing technology is used to remove their toxicity and optimize their adjuvant performance, such as from LT to dmLT (R192G/L211A) and from CT to mmCT. On the other hand, the biogenic adjuvants could be fused with the target antigens by DNA recombination technology to construct the fusion protein vaccines. In addition, most biogenic adjuvants could be encoded and expressed in beneficial bacterial strains, such as probiotics (e.g., LAB), attenuated live bacteria, or gut commensal bacteria, as a protective strategy across the GIT against degradation from gastric acid and proteases, etc. This is also another promising way to develop oral vaccines. Ideally, adjuvants should not induce adaptive immune responses against themselves, but should promote appropriate immune response to accompanying antigens [190, 191]. However, some well-known protein-based oral adjuvants listed above, including FliC [192, 193], CTB [194], FnBPA [51], PorA [195], and even the well-studied dmLT [196], have been reported to produce a certain degree of immune response against themselves in the host, thereby potentially affecting their effectiveness as adjuvants. However, FljB has been reported to produce no immune response against itself [40], and an appropriate oral dose of dmLT is still safe, well tolerated, and reasonably immunogenic [196].
Using non-biogenic adjuvants, protein antigens could be coated in a variety of ways, such as lipidation, nanoparticle encapsulation (using polymersomes, for example), adsorption and conjugation to polymer-based microparticles/nanoparticles (using PLGA, chitosan, alginate, etc.) and/or additive/synergistic admixture [167]. In addition, combining two or more types of adjuvant materials (including biogenic and non-biogenic materials) to construct composite adjuvants can make up for inherent flaws of some biogenic adjuvants, such as their short half-life and ease of degradation in GIT, and strengthen their immune effects in the intestinal tract. There are also some nanomaterials that can control the slow release of vaccines. For example, some TLR ligands are often chimeric with other nano-adjuvant materials, therefore the adjuvant effect is better.
Undoubtedly, in order to exert the adjuvant activity for peroral mucosal vaccines, more attention should be focused on the endogenous immune activation mechanisms of adjuvants and the immunological and pharmacological relationship among adjuvants, vaccines (or antigens) and the host gastrointestinal mucosal immune system. Although there are various adjuvant strategies, they should all be studied in detail before selecting the optimum formulation. The formulation of vaccines and adjuvants should not only maintain the immunogenicity of the vaccine but also protect their adjuvant activity. This is an issue that needs meticulous consideration when designing oral vaccines.
The development of oral adjuvants still presents many challenges. As mentioned earlier, the GIT is a complex and harsh environment, which leads to the instability of adjuvants, especially biogenic adjuvants, and hinders the interaction between oral adjuvants and intestinal epithelial cells. On the other hand, the low proportion of M cells in the intestinal epithelium would limit the effect of M cell-mediated adjuvants. However, we believe that more and more oral adjuvants, similar to RANKL, that can induce M-cell differentiation will emerge in the future to change this dilemma. The potential safety concerns of adjuvants, such as cytokine-derived adjuvants [93], are another challenge. The effective targeting, pharmacokinetics and nanotoxicology of some potential oral adjuvants need to be further evaluated.
As mentioned previously, the targeting peptides that target intestinal immune cells (or receptor proteins on their cell surface) can improve the binding ability of antigens to bind to intestinal DCs or M cells (or receptors). Therefore, in addition to the targeting peptides of human and/or mouse intestinal immune cells (and their receptor proteins), some researchers are trying to screen and identify specific M cells or DC-binding peptides of other animal species for studies in veterinary or comparative medicine by using the cell-based phage display technique combined with high-throughput sequencing; for instance, the chicken DC-binding peptide (SPHLHTSSPWER, named SP) [197] and the porcine TLR2-targeting peptide ligand (NAGHLSQ) [198] [porcine TLR2 is highly expressed in M cells and plays an important role in pig mucosal immune responses]. Aiming at M cells or DCs (or receptor proteins on their cell surface), it will be a trend to develop more targeting peptides for different animal species, especially in the prevention and control of veterinary infectious diseases. Furthermore, with further understanding of the mechanisms of action of some less-studied candidate adjuvants, such as muramyl dipeptide and tuftsin fusion protein (MT) [54, 55], N. meningitidis PorA [57], c-di-AMP [58], RCK protein [62], etc., these may be the future development direction of oral adjuvants.
With the development of oral adjuvants in recent years, it is believed that more reasonable and effective oral adjuvants will appear in the future and hence solve the challenges mentioned.
Acknowledgements
The authors acknowledge the assistance of Xiaojie Chen, Leilei Liu and Tingting Shen from Zhaoqing University in the preparation and revision of this article.
Declarations
Funding
Financial support for this study was provided by the National Natural Science Foundation of China (Grant no. 32102703), Basic and Applied Basic Research Joint Fund of Guangdong Province (2019A1515111186), National College Students’ Innovation and Entrepreneurship Training Plan Program in 2021 (202110580008), College Students’ Innovation and Entrepreneurship Training Plan Program of Guangdong Province (S202010580051, S202210580052), Fund of Construction of Ecological Poultry Industry Technology System in Guizhou Province (Functional Laboratory of Disease Prevention and Control), and the Scientific Research Start-up Fund of Zhaoqing University (611/180160), Social Public Welfare Science and Technology Research Project of Zhongshan City (2019B2022).
Conflict of interest
Bingming Ou, Ying Yang, Haihui Lv, Xin Lin, and Minyu Zhang declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
BO Conceived the article. BO, YY and MZ prepared a draft and edited this review as well as subsequent revisions. All authors contributed to the preparation of this manuscript.
Footnotes
Bingming Ou and Ying Yang contributed equally to this work.
References
- 1.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 2.Jin Z, Gao S, Cui X, Sun D, Zhao K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int J Pharm. 2019;572:118731. doi: 10.1016/j.ijpharm.2019.118731. [DOI] [PubMed] [Google Scholar]
- 3.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 4.Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30(32):4807–4812. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Davitt CJ, Lavelle EC. Delivery strategies to enhance oral vaccination against enteric infections. Adv Drug Deliv Rev. 2015;91:52–69. doi: 10.1016/j.addr.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez JEV, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv Drug Deliv Rev. 2017;114:116–131. doi: 10.1016/j.addr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talaat M, Kandeel A, El-Shoubary W, Bodenschatz C, Khairy I, Oun S, et al. Occupational exposure to needlestick injuries and hepatitis B vaccination coverage among health care workers in Egypt. Am J Infect Control. 2003;31(8):469–474. doi: 10.1016/j.ajic.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Coppel RL. Oral vaccine delivery: can it protect against non-mucosal pathogens? Expert Rev Vaccines. 2008;7(6):729–738. doi: 10.1586/14760584.7.6.729. [DOI] [PubMed] [Google Scholar]
- 9.Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasetti MF, Simon JK, Sztein MB, Levine MM. Immunology of gut mucosal vaccines. Immunol Rev. 2011;239(1):125–148. doi: 10.1111/j.1600-065X.2010.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev. 2011;241(1):241–259. doi: 10.1111/j.1600-065X.2011.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park T-E, Singh B, Maharjan S, Jiang T, Yoon S-Y, Kang S-K, et al. Mucosal delivery of vaccine by M cell targeting strategies. Curr Drug Ther. 2014;9(1):9–20. doi: 10.2174/1574885509666140805004042. [DOI] [Google Scholar]
- 13.Abautret-Daly AE, Davitt CJ, Lavelle EC. Harnessing the antibacterial and immunological properties of mucosal-associated invariant T cells in the development of novel oral vaccines against enteric infections. Biochem Pharmacol. 2014;92(2):173–183. doi: 10.1016/j.bcp.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Davitt CJ, Longet S, Albutti A, Aversa V, Nordqvist S, Hackett B, et al. Alpha-galactosylceramide enhances mucosal immunity to oral whole-cell cholera vaccines. Mucosal Immunol. 2019;12(4):1055–1064. doi: 10.1038/s41385-019-0159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lycke N, Lebrero-Fernández C. ADP-ribosylating enterotoxins as vaccine adjuvants. Curr Opin Pharmacol. 2018;41:42–51. doi: 10.1016/j.coph.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Freytag L, Clements J. Mucosal adjuvants. Vaccine. 2005;23(15):1804–1813. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Norton EB, Lawson LB, Freytag LC, Clements JD. Characterization of a mutant Escherichia coli heat-labile toxin, LT (R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol. 2011;18(4):546–551. doi: 10.1128/CVI.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larena M, Holmgren J, Lebens M, Terrinoni M, Lundgren A. Cholera toxin, and the related nontoxic adjuvants mmCT and dmLT, promote human Th17 responses via cyclic AMP-protein kinase A and inflammasome-dependent IL-1 signaling. J Immunol. 2015;194(8):3829. doi: 10.4049/jimmunol.1401633. [DOI] [PubMed] [Google Scholar]
- 19.Norton EB, Lawson LB, Mahdi Z, Freytag LC, Clements JD. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect Immun. 2012;80(7):2426–2435. doi: 10.1128/IAI.00181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anosova N, Chabot S, Shreedhar V, Borawski J, Dickinson B, Neutra M. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer's patches. Mucosal Immunol. 2008;1(1):59–67. doi: 10.1038/mi.2007.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leach S, Clements JD, Kaim J, Lundgren A. The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PLoS One. 2012;7(12):e51718. doi: 10.1371/journal.pone.0051718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Kamary SS, Cohen MB, Bourgeois AL, Van DV, Bauers LN, Reymann M, et al. Safety and immunogenicity of a single oral dose of recombinant double mutant heat-labile toxin derived from enterotoxigenic Escherichia coli. Clin Vaccine Immunol Cvi. 2013;20(11):1764–1770. doi: 10.1128/CVI.00464-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, et al. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol CVI. 2010;17(6):1005–1012. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summerton NA, Welch RW, Bondoc L, Yang HH, Pleune B, Ramachandran N, et al. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin. Vaccine. 2010;28(5):1404–1411. doi: 10.1016/j.vaccine.2009.10.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ottsjö LS, Flach CF, Clements J, Holmgren J, Raghavan S. A Double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect Immun. 2013;81:1532–1540. doi: 10.1128/IAI.01407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alo. 2013. [DOI] [PubMed]
- 27.Guillobel HC, Carinhanha JI, Cárdenas L, Clements JD, Almeida DF, De Ferreira LC. Adjuvant activity of a nontoxic mutant of Escherichia coli heat-labile enterotoxin on systemic and mucosal immune responses elicited against a heterologous antigen carried by a live Salmonella enterica Serovar Typhimurium vaccine strain. Infect Immun. 2000;68(7):4349–4353. doi: 10.1128/IAI.68.7.4349-4353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, et al. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine. 2013;31(20):2457–2464. doi: 10.1016/j.vaccine.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, et al. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli (ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled phase I study. Vaccine. 2014;32(52):7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 30.Harro C, Bourgeois AL, Sack D, Walker R, DeNearing B, Brubaker J, et al. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine. 2019;37(14):1978–1986. doi: 10.1016/j.vaccine.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebens M, Terrinoni M, Karlsson SL, Larena M, Gustafsson-Hedberg T, Källgård S, et al. Construction and preclinical evaluation of mmCT, a novel mutant cholera toxin adjuvant that can be efficiently produced in genetically manipulated Vibrio cholerae. Vaccine. 2016;34(18):2121–2128. doi: 10.1016/j.vaccine.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Holmgren J, Nordqvist S, Blomquist M, Jeverstam F, Lebens M, Raghavan S. Preclinical immunogenicity and protective efficacy of an oral Helicobacter pylori inactivated whole cell vaccine and multiple mutant cholera toxin: a novel and non-toxic mucosal adjuvant. Vaccine. 2018;36(41):6223–6230. doi: 10.1016/j.vaccine.2018.07.073. [DOI] [PubMed] [Google Scholar]
- 33.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Zhou M, Zhang G, Ren G, Gnanadurai CW, Li Z, Chai Q, et al. Recombinant rabies viruses expressing GM-CSF or flagellin are effective vaccines for both intramuscular and oral immunizations. PLoS One. 2013;8(5):e63384. doi: 10.1371/journal.pone.0063384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat Immunol. 2006;7(6):569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 36.Vilander AC, Dean GA. Adjuvant strategies for lactic acid bacterial mucosal vaccines. Vaccines. 2019;7(4):150. doi: 10.3390/vaccines7040150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clin Exp Vaccine Res. 2012;1(1):50. doi: 10.7774/cevr.2012.1.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui B, Liu X, Fang Y, Zhou P, Zhang Y, Wang Y. Flagellin as a vaccine adjuvant. Expert Rev Vaccines. 2018;17(4):335–349. doi: 10.1080/14760584.2018.1457443. [DOI] [PubMed] [Google Scholar]
- 39.Ren Z, Zhao Y, Liu J, Ji X, Meng L, Wang T, et al. Inclusion of membrane-anchored LTB or flagellin protein in H5N1 virus-like particles enhances protective responses following intramuscular and oral immunization of mice. Vaccine. 2018;36(40):5990–5998. doi: 10.1016/j.vaccine.2018.08.053. [DOI] [PubMed] [Google Scholar]
- 40.Girard A, Saron W, Bergeron-Sandoval L-P, Sarhan F, Archambault D. Flagellin produced in plants is a potent adjuvant for oral immunization. Vaccine. 2011;29(38):6695–6703. doi: 10.1016/j.vaccine.2011.06.092. [DOI] [PubMed] [Google Scholar]
- 41.Hajam IA, Kim JH, Lee JH. Incorporation of membrane-anchored flagellin into Salmonella Gallinarum bacterial ghosts induces early immune responses and protection against fowl typhoid in young layer chickens. Vet Immunol Immunopathol. 2018;199:61–69. doi: 10.1016/j.vetimm.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Eom JS, Kim JS, Im Jang J, Kim B-H, Yoo SY, Choi JH, et al. Enhancement of host immune responses by oral vaccination to Salmonella enterica serovar Typhimurium harboring both FliC and FljB flagella. PLoS One. 2013;8(9):e74850. doi: 10.1371/journal.pone.0074850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto S, Kutsukake K. FljA-mediated posttranscriptional control of phase 1 flagellin expression in flagellar phase variation of Salmonella enterica serovar Typhimurium. J Bacteriol. 2006;188(3):958–967. doi: 10.1128/JB.188.3.958-967.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y, Yang Y, Ou B, Xia P, Zhou M, Li L, et al. The flagellin hypervariable region is a potential flagella display domain in probiotic Escherichia coli strain Nissle 1917. Arch Microbiol. 2016;198(7):603–610. doi: 10.1007/s00203-016-1219-3. [DOI] [PubMed] [Google Scholar]
- 45.Wu JY, Newton S, Judd A, Stocker B, Robinson WS. Expression of immunogenic epitopes of hepatitis B surface antigen with hybrid flagellin proteins by a vaccine strain of Salmonella. Proc Natl Acad Sci. 1989;86(12):4726–4730. doi: 10.1073/pnas.86.12.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chauhan N, Kumar R, Badhai J, Preet A, Yadava PK. Immunogenicity of cholera toxin B epitope inserted in Salmonella flagellin expressed on bacteria and administered as DNA vaccine. Mol Cell Biochem. 2005;276(1–2):1–6. doi: 10.1007/s11010-005-2240-z. [DOI] [PubMed] [Google Scholar]
- 47.Braga CJ, Massis LM, Sbrogio-Almeida ME, Alencar BC, Bargieri DY, Boscardin SB, et al. CD8+ T cell adjuvant effects of Salmonella FliCd flagellin in live vaccine vectors or as purified protein. Vaccine. 2010;28(5):1373–1382. doi: 10.1016/j.vaccine.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Gaillard J-L, Berche P, Frehel C, Gouln E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65(7):1127–1141. doi: 10.1016/0092-8674(91)90009-N. [DOI] [PubMed] [Google Scholar]
- 49.Innocentin S, Guimarães V, Miyoshi A, Azevedo V, Langella P, Chatel J-M, et al. Lactococcus lactis expressing either Staphylococcus aureus fibronectin-binding protein A or Listeria monocytogenes internalin A can efficiently internalize and deliver DNA in human epithelial cells. Appl Environ Microbiol. 2009;75(14):4870–4878. doi: 10.1128/AEM.00825-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Azevedo M, Karczewski J, Lefévre F, Azevedo V, Miyoshi A, Wells JM, et al. In vitro and in vivo characterization of DNA delivery using recombinant Lactococcus lactis expressing a mutated form of L. monocytogenes Internalin A. BMC Microbiol. 2012;12(1):1–9. doi: 10.1186/1471-2180-12-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J, Yang G, Gao X, Zhang Z, Liu Y, Liu Q, et al. Recombinant invasive Lactobacillus plantarum expressing fibronectin binding protein A induce specific humoral immune response by stimulating differentiation of dendritic cells. Benef Microbes. 2019;10(5):589–604. doi: 10.3920/BM2018.0157. [DOI] [PubMed] [Google Scholar]
- 52.Pontes D, Innocentin S, Del Carmen S, Almeida JF, LeBlanc J-G, de Moreno de LeBlanc A, et al. Production of fibronectin binding protein A at the surface of Lactococcus lactis increases plasmid transfer in vitro and in vivo. 2012. [DOI] [PMC free article] [PubMed]
- 53.Pereira VB, Saraiva TDL, Souza BM, Zurita-Turk M, Azevedo MSP, De Castro CP, et al. Development of a new DNA vaccine based on mycobacterial ESAT-6 antigen delivered by recombinant invasive Lactococcus lactis FnBPA+ Appl Microbiol Biotechnol. 2015;99(4):1817–1826. doi: 10.1007/s00253-014-6285-3. [DOI] [PubMed] [Google Scholar]
- 54.Jiang X, Yu M, Qiao X, Liu M, Tang L, Jiang Y, et al. Up-regulation of MDP and tuftsin gene expression in Th1 and Th17 cells as an adjuvant for an oral Lactobacillus casei vaccine against anti-transmissible gastroenteritis virus. Appl Microbiol Biotechnol. 2014;98(19):8301–8312. doi: 10.1007/s00253-014-5893-2. [DOI] [PubMed] [Google Scholar]
- 55.Wardowska A, Dzierzbicka K, Menderska A, Trzonkowski P. New conjugates of tuftsin and muramyl dipeptide as stimulators of human monocyte-derived dendritic cells. Protein Pept Lett. 2013;20(2):200–204. doi: 10.2174/092986613804725299. [DOI] [PubMed] [Google Scholar]
- 56.Derrick JP, Urwin R, Suker J, Feavers IM, Maiden MC. Structural and evolutionary inference from molecular variation in Neisseria porins. Infect Immun. 1999;67(5):2406–2413. doi: 10.1128/IAI.67.5.2406-2413.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasquez AE, Manzo RA, Soto DA, Barrientos MJ, Maldonado AE, Mosqueira M, et al. Oral administration of recombinant Neisseria meningitidis PorA genetically fused to H. pylori HpaA antigen increases antibody levels in mouse serum, suggesting that PorA behaves as a putative adjuvant. Hum Vaccines Immunother. 2015;11(3):776–788. doi: 10.1080/21645515.2015.1011011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quintana I, Espariz M, Villar SR, González FB, Pacini MF, Cabrera G, et al. Genetic engineering of Lactococcus lactis co-producing antigen and the mucosal adjuvant 3′ 5′-cyclic di adenosine monophosphate (c-di-AMP) as a design strategy to develop a mucosal vaccine prototype. Front Microbiol. 2018;9:2100. doi: 10.3389/fmicb.2018.02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Škrnjug I, Rueckert C, Libanova R, Lienenklaus S, Weiss S, Guzmán CA. The mucosal adjuvant cyclic di-AMP exerts immune stimulatory effects on dendritic cells and macrophages. PLoS One. 2014;9(4):e95728. doi: 10.1371/journal.pone.0095728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heffernan E, Reed S, Hackett J, Fierer J, Roudier C, Guiney D. Mechanism of resistance to complement-mediated killing of bacteria encoded by the Salmonella typhimurium virulence plasmid gene rck. J Clin Investig. 1992;90(3):953–964. doi: 10.1172/JCI115972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosselin M, Virlogeux-Payant I, Roy C, Bottreau E, Sizaret P-Y, Mijouin L, et al. Rck of Salmonella enterica, subspecies enterica serovar enteritidis, mediates zipper-like internalization. Cell Res. 2010;20(6):647–664. doi: 10.1038/cr.2010.45. [DOI] [PubMed] [Google Scholar]
- 62.Wang W, Song Y, Liu L, Zhang Y, Wang T, Zhang W, et al. Neutralizing-antibody-mediated protection of chickens against infectious bursal disease via one-time vaccination with inactivated recombinant Lactococcus lactis expressing a fusion protein constructed from the RCK protein of Salmonella enterica and VP2 of infectious bursal disease virus. Microb Cell Fact. 2019;18(1):1–12. doi: 10.1186/s12934-019-1061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serradell MC, Rupil LL, Martino RA, Prucca CG, Carranza PG, Saura A, et al. Efficient oral vaccination by bioengineering virus-like particles with protozoan surface proteins. Nat Commun. 2019;10(1):1–15. doi: 10.1038/s41467-018-08265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bringer M-A, Darfeuille-Michaud A. Bacterial adhesion to intestinal mucosa, in mucosal immunology. New York: Elsevier; 2015. pp. 949–953. [Google Scholar]
- 65.Kyd JM, Cripps AW. Functional differences between M cells and enterocytes in sampling luminal antigens. Vaccine. 2008;26(49):6221–6224. doi: 10.1016/j.vaccine.2008.09.061. [DOI] [PubMed] [Google Scholar]
- 66.Chamcha V, Jones A, Quigley BR, Scott JR, Amara RR. Oral immunization with a recombinant Lactococcus lactis—expressing HIV-1 antigen on group A Streptococcus pilus induces strong mucosal immunity in the gut. J Immunol. 2015;195(10):5025–5034. doi: 10.4049/jimmunol.1501243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez-Hernandez MB, Liu T, Payne HC, Stencel-Baerenwald JE, Ikizler M, Yagita H, et al. Efficient norovirus and reovirus replication in the mouse intestine requires microfold (M) cells. J Virol. 2014;88(12):6934–6943. doi: 10.1128/JVI.00204-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu M, Yang Y, Zhu C, Guo S, Gan Y. Advances in the transepithelial transport of nanoparticles. Drug Discov Today. 2016;21(7):1155–1161. doi: 10.1016/j.drudis.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Niess JH, Reinecker H-C. Lamina propria dendritic cells in the physiology and pathology of the gastrointestinal tract. Curr Opin Gastroenterol. 2005;21(6):687–691. doi: 10.1097/01.mog.0000181710.96904.58. [DOI] [PubMed] [Google Scholar]
- 70.Mohamadzadeh M, Duong T, Sandwick S, Hoover T, Klaenhammer T. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc Natl Acad Sci. 2009;106(11):4331–4336. doi: 10.1073/pnas.0900029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2(4):361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 72.Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci. 2005;102(8):2880–2885. doi: 10.1073/pnas.0500098102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Islam MA, Firdous J, Badruddoza AZM, Reesor E, Azad M, Hasan A, et al. M cell targeting engineered biomaterials for effective vaccination. Biomaterials. 2019;192:75–94. doi: 10.1016/j.biomaterials.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 74.Miller H, Zhang J, KuoLee R, Patel GB, Chen W. Intestinal M cells: the fallible sentinels? World J Gastroenterol WJG. 2007;13(10):1477. doi: 10.3748/wjg.v13.i10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li HS, Piao D-C, Jiang T, Bok J-D, Cho C-S, Lee Y-S, et al. Recombinant interleukin 6 with M cell-targeting moiety produced in Lactococcus lactis IL1403 as a potent mucosal adjuvant for peroral immunization. Vaccine. 2015;33(16):1959–1967. doi: 10.1016/j.vaccine.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 76.Lelouard H, Fallet M, de Bovis B, Méresse S, Gorvel JP. Peyer's patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology. 2012;142(3):592–601. doi: 10.1053/j.gastro.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 77.Yoo M-K, Kang S-K, Choi J-H, Park I-K, Na H-S, Lee H-C, et al. Targeted delivery of chitosan nanoparticles to Peyer’s patch using M cell-homing peptide selected by phage display technique. Biomaterials. 2010;31(30):7738–7747. doi: 10.1016/j.biomaterials.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 78.Kim S-H, Seo K-W, Kim J, Lee K-Y, Jang Y-S. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol. 2010;185(10):5787–5795. doi: 10.4049/jimmunol.0903184. [DOI] [PubMed] [Google Scholar]
- 79.Wang X, Wang L, Zheng D, Chen S, Shi W, Qiao X, et al. Oral immunization with a Lactobacillus casei-based anti-porcine epidemic diarrhoea virus (PEDV) vaccine expressing microfold cell-targeting peptide Co1 fused with the COE antigen of PEDV. J Appl Microbiol. 2018;124(2):368–378. doi: 10.1111/jam.13652. [DOI] [PubMed] [Google Scholar]
- 80.Oh S-H, Kim S-H, Jeon J-H, Kim EB, Lee N-K, Beck S, et al. Cytoplasmic expression of a model antigen with M Cell-targeting moiety in lactic acid bacteria and implication of the mechanism as a mucosal vaccine via oral route. Vaccine. 2021;39:4072–4081. doi: 10.1016/j.vaccine.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 81.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature. 2009;462(7270):226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 82.Khan IU, Huang J, Liu R, Wang J, Xie J, Zhu N. Phage display-derived ligand for mucosal transcytotic receptor GP-2 promotes antigen delivery to m cells and induces antigen-specific immune response. SLAS Discov Adv Life Sci R&D. 2017;22(7):879–886. doi: 10.1177/2472555217690483. [DOI] [PubMed] [Google Scholar]
- 83.Kim JI, Park TE, Maharjan S, Li HS, Lee HB, Kim IS, et al. Soluble RANKL expression in Lactococcus lactis and investigation of its potential as an oral vaccine adjuvant. BMC Immunol. 2015;16(1):1–11. doi: 10.1186/s12865-015-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor RT, Patel SR, Lin E, Butler BR, Lake JG, Newberry RD, et al. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J Immunol. 2007;178(9):5659–5667. doi: 10.4049/jimmunol.178.9.5659. [DOI] [PubMed] [Google Scholar]
- 85.Maharjan S, Singh B, Jiang T, Yoon S-Y, Li H-S, Kim G, et al. Systemic administration of RANKL overcomes the bottleneck of oral vaccine delivery through microfold cells in ileum. Biomaterials. 2016;84:286–300. doi: 10.1016/j.biomaterials.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 86.Choe S, Song S, Piao D, Park G-N, Shin J, Choi YJ, et al. Efficacy of orally administered porcine epidemic diarrhea vaccine-loaded hydroxypropyl methylcellulose phthalate microspheres and RANKL-secreting L. lactis. Vet Microbiol. 2020;242:108604. doi: 10.1016/j.vetmic.2020.108604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Macri C, Dumont C, Johnston AP, Mintern JD. Targeting dendritic cells: a promising strategy to improve vaccine effectiveness. Clin Transl Immunol. 2016;5(3):e66. doi: 10.1038/cti.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Owen JL, Sahay B, Mohamadzadeh M. New generation of oral mucosal vaccines targeting dendritic cells. Curr Opin Chem Biol. 2013;17(6):918–924. doi: 10.1016/j.cbpa.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Øverland L. Secretion and anchoring of proteins in Lactobacillus plantarum: Studies of a dendritic cell-targeted Mycobacterium tuberculosis antigen. Norwegian University of Life Sciences, Ås; 2013.
- 90.Hou X, Jiang X, Jiang Y, Tang L, Xu Y, Qiao X, et al. Oral immunization against PEDV with recombinant Lactobacillus casei expressing dendritic cell-targeting peptide fusing COE protein of PEDV in piglets. Viruses. 2018;10(3):106. doi: 10.3390/v10030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X, Wang L, Huang X, Ma S, Yu M, Shi W, et al. Oral delivery of probiotics expressing dendritic cell-targeting peptide fused with porcine epidemic diarrhea virus COE antigen: a promising vaccine strategy against PEDV. Viruses. 2017;9(11):312. doi: 10.3390/v9110312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi S-H, Yang W-T, Yang G-L, Zhang X-K, Liu Y-Y, Zhang L-J, et al. Lactobacillus plantarum vaccine vector expressing hemagglutinin provides protection against H9N2 challenge infection. Virus Res. 2016;211:46–57. doi: 10.1016/j.virusres.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 93.Newsted D, Fallahi F, Golshani A, Azizi A. Advances and challenges in mucosal adjuvant technology. Vaccine. 2015;33(21):2399–2405. doi: 10.1016/j.vaccine.2015.03.096. [DOI] [PubMed] [Google Scholar]
- 94.Tovey MG, Lallemand C. Adjuvant activity of cytokines. Vaccine Adjuv. 2010;626:287–309. [DOI] [PubMed]
- 95.Kajikawa A, Masuda K, Katoh M, Igimi S. Adjuvant effects for oral immunization provided by recombinant Lactobacillus casei secreting biologically active murine interleukin-1β. Clin Vaccine Immunol. 2010;17(1):43–48. doi: 10.1128/CVI.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Potocki W, Negri A, Peszyńska-Sularz G, Hinc K, Obuchowski M, Iwanicki A. IL-1 fragment modulates immune response elicited by recombinant Bacillus subtilis spores presenting an antigen/adjuvant chimeric protein. Mol Biotechnol. 2018;60(11):810–819. doi: 10.1007/s12033-018-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng Z, Geng Y, Wang K, Yu Z, Yang PO, Yang Z, et al. Adjuvant effects of interleukin-2 co-expression with VP60 in an oral vaccine delivered by attenuated Salmonella typhimurium against rabbit hemorrhagic disease. Vet Microbiol. 2019;230:49–55. doi: 10.1016/j.vetmic.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H-X, Qiu Y-Y, Zhao Y-H, Liu X-T, Liu M, Yu A-L. Immunogenicity of oral vaccination with Lactococcus lactis derived vaccine candidate antigen (UreB) of Helicobacter pylori fused with the human interleukin 2 as adjuvant. Mol Cell Probes. 2014;28(1):25–30. doi: 10.1016/j.mcp.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 99.Hinc K, Stasiłojć M, Piątek I, Peszyńska-Sularz G, Isticato R, Ricca E, et al. Mucosal adjuvant activity of IL-2 presenting spores of Bacillus subtilis in a murine model of Helicobacter pylori vaccination. PLoS One. 2014;9(4):e95187. doi: 10.1371/journal.pone.0095187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hugentobler F, Di Roberto RB, Gillard J, Cousineau B. Oral immunization using live Lactococcus lactis co-expressing LACK and IL-12 protects BALB/c mice against Leishmania major infection. Vaccine. 2012;30(39):5726–5732. doi: 10.1016/j.vaccine.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 101.Chung J-Y, Sung E-J, Cho C-G, Seo K-W, Lee J-S, Bhang D-H, et al. Effect of recombinant Lactobacillus expressing canine GM-CSF on immune function in dogs. J Microbiol Biotechnol. 2009;19(11):1401–1407. doi: 10.4014/jmb.0904.4010. [DOI] [PubMed] [Google Scholar]
- 102.Xu Y-G, Guan X-T, Liu Z-M, Tian C-Y, Cui L-C. Immunogenicity in swine of orally administered recombinant Lactobacillus plantarum expressing classical swine fever virus E2 protein in conjunction with thymosin α-1 as an adjuvant. Appl Environ Microbiol. 2015;81(11):3745–3752. doi: 10.1128/AEM.00127-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li CL, Zhang T, Saibara T, Nemoto Y, Ono M, Akisawa N, et al. Thymosin α1 accelerates restoration of T cell-mediated neutralizing antibody response in immunocompromised hosts. Int Immunopharmacol. 2002;2(1):39–46. doi: 10.1016/S1567-5769(01)00136-9. [DOI] [PubMed] [Google Scholar]
- 104.Jiang Y, Ma Z, Zhao P, Pan Y, Liu Y, Feng J, et al. Effect of Thymosin-α1 on T-helper 1 Cell and T-helper 2 cell cytokine synthesis in patients with hepatitis B virus e antigen-positive chronic hepatitis B. J Int Med Res. 2010;38(6):2053–2062. doi: 10.1177/147323001003800620. [DOI] [PubMed] [Google Scholar]
- 105.Czajkowsky DM, Hu J, Shao Z, Pleass RJ. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med. 2012;4(10):1015–1028. doi: 10.1002/emmm.201201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Konduru K, Bradfute SB, Jacques J, Manangeeswaran M, Nakamura S, Morshed S, et al. Ebola virus glycoprotein Fc fusion protein confers protection against lethal challenge in vaccinated mice. Vaccine. 2011;29(16):2968–2977. doi: 10.1016/j.vaccine.2011.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu H, Saxena A, Sidhu SS, Wu D. Fc engineering for developing therapeutic bispecific antibodies and novel scaffolds. Front Immunol. 2017;8:38. doi: 10.3389/fimmu.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sockolosky JT, Szoka FC. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv Drug Deliv Rev. 2015;91:109–124. doi: 10.1016/j.addr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol. 2011;29(2):158–163. doi: 10.1038/nbt.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, Li X, Hao G, Zhang H, Yang H, Chen H, et al. Fusion of pseudorabies virus glycoproteins to IgG Fc enhances protective immunity against pseudorabies virus. Virology. 2019;536:49. doi: 10.1016/j.virol.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 111.Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, et al. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85(20):10542–10553. doi: 10.1128/JVI.05441-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang W-T, Yang G-L, Wang Q, Huang H-B, Jiang Y-L, Shi C-W, et al. Protective efficacy of Fc targeting conserved influenza virus M2e antigen expressed by Lactobacillus plantarum. Antivir Res. 2017;138:9–21. doi: 10.1016/j.antiviral.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 113.Li J, Li X, Ma H, Ren X, Hao G, Zhang H, et al. Efficient mucosal vaccination of a novel classical swine fever virus E2-Fc fusion protein mediated by neonatal Fc receptor. Vaccine. 2020;38(29):4574–4583. doi: 10.1016/j.vaccine.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 114.Ma S, Wang L, Huang X, Wang X, Chen S, Shi W, et al. Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb Cell Fact. 2018;17(1):1–12. doi: 10.1186/s12934-018-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carter D, Reed SG. Role of adjuvants in modeling the immune response. Curr Opin HIV AIDS. 2010;5(5):409. doi: 10.1097/COH.0b013e32833d2cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith A, Perelman M, Hinchcliffe M. Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum Vaccin Immunother. 2014;10(3):797–807. doi: 10.4161/hv.27449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kapusta J, Pniewski T, Wojciechowicz J, Bociąg P, Płucienniczak A. Nanogram doses of alum-adjuvanted HBs antigen induce humoral immune response in mice when orally administered. Arch Immunol Ther Exp. 2010;58(2):143–151. doi: 10.1007/s00005-010-0065-2. [DOI] [PubMed] [Google Scholar]
- 118.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30(1):23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 119.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol. 2011;9(1):1–11. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng J, Gao S, Cui X, Sun D, Zhao K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int J Pharm. 2019;572:118731. doi: 10.1016/j.ijpharm.2019.118731. [DOI] [PubMed] [Google Scholar]
- 121.Basu A, Domb AJ. Recent advances in polyanhydride based biomaterials. Adv Mater. 2018;30(41):1706815. doi: 10.1002/adma.201706815. [DOI] [PubMed] [Google Scholar]
- 122.Rowe RC, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipients. London: Pharmaceutical Press and American Pharmacist Association; 2005. [Google Scholar]
- 123.Rowe RC, Sheskey PJ, Owen S. Handbook of pharmaceutical excipients. Washington, DC: Pharmaceutical Press and American Pharmacists Association; 2009. [Google Scholar]
- 124.Karolewicz B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm J. 2016;24:525–536. doi: 10.1016/j.jsps.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Renu S, Markazi AD, Dhakal S, Lakshmanappa YS, Gourapura SR, Shanmugasundaram R, et al. Surface engineered polyanhydride-based oral Salmonella subunit nanovaccine for poultry. Int J Nanomed. 2018;13:8195. doi: 10.2147/IJN.S185588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tamayo I, Irache JM, Mansilla C, Ochoa-Reparaz J, Lasarte JJ, Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin Vaccine Immunol. 2010;17(9):1356–1362. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ramirez JV, Tygrett LT, Hao J, Habte HH, Cho MW, Greenspan NS, et al. Polyanhydride nanovaccines induce germinal center B Cell formation and sustained serum antibody responses. J Biomed Nanotechnol. 2016;12(6):1303–1311. doi: 10.1166/jbn.2016.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Camacho AI, Da Costa MR, Tamayo I, de Souza J, Lasarte JJ, Mansilla C, et al. Poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine. 2011;29(41):7130–7135. doi: 10.1016/j.vaccine.2011.05.072. [DOI] [PubMed] [Google Scholar]
- 129.Salman HH, Irache JM, Gamazo C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine. 2009;27(35):4784–4790. doi: 10.1016/j.vaccine.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 130.Gómez S, Gamazo C, Roman BS, Ferrer M, Sanz ML, Irache JM. Gantrez AN nanoparticles as an adjuvant for oral immunotherapy with allergens. Vaccine. 2007;25(29):5263–5271. doi: 10.1016/j.vaccine.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 131.Salman HH, Gamazo C, Campanero MA, Irache JM. Salmonella-like bioadhesive nanoparticles. J Control Release. 2005;106(1–2):1–13. doi: 10.1016/j.jconrel.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 132.Agüeros M, Areses P, Campanero MA, Salman H, Quincoces G, Peñuelas I, et al. Bioadhesive properties and biodistribution of cyclodextrin–poly (anhydride) nanoparticles. Eur J Pharm Sci. 2009;37(3–4):231–240. doi: 10.1016/j.ejps.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 133.Stern M, Ulrich K, Geddes D, Alton E. Poly (D, L-lactide-co-glycolide)/DNA microspheres to facilitate prolonged transgene expression in airway epithelium in vitro, ex vivo and in vivo. Gene Ther. 2003;10(16):1282–1288. doi: 10.1038/sj.gt.3301994. [DOI] [PubMed] [Google Scholar]
- 134.O’Hagan T, Singh DM, Gupta RK. Poly (lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv Drug Deliv Rev. 1998;32(3):225–246. doi: 10.1016/S0169-409X(98)00012-X. [DOI] [PubMed] [Google Scholar]
- 135.Chuang S-C, Ko J-C, Chen C-P, Du J-T, Yang C-D. Encapsulation of chimeric protein rSAG1/2 into poly (lactide-co-glycolide) microparticles induces long-term protective immunity against Toxoplasma gondii in mice. Exp Parasitol. 2013;134(4):430–437. doi: 10.1016/j.exppara.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 136.Kim SY, Doh HJ, Jang MH, Ha YJ, Chung SI, Park HJ. Oral immunization with Helicobacter pylori-loaded poly (d,l-lactide-co-glycolide) nanoparticles. Helicobacter. 1999;4(1):33–39. doi: 10.1046/j.1523-5378.1999.09046.x. [DOI] [PubMed] [Google Scholar]
- 137.Kofler N, Ruedl C, Rieser C, Wick G, Wolf H. Oral immunization with poly-(d,l-lactide-co-glycolide) and poly-(l-lactic acid) microspheres containing pneumotropic bacterial antigens. Int Arch Allergy Immunol. 1997;113(4):424–431. doi: 10.1159/000237618. [DOI] [PubMed] [Google Scholar]
- 138.Ramya R, Verma P, Chaturvedi V, Gupta P, Pandey K, Madhanmohan M, et al. Poly (lactide-co-glycolide) microspheres: a potent oral delivery system to elicit systemic immune response against inactivated rabies virus. Vaccine. 2009;27(15):2138–2143. doi: 10.1016/j.vaccine.2009.01.129. [DOI] [PubMed] [Google Scholar]
- 139.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Saltzman WM, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27(23):3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saurabh D, Kiran A, Srinivas M, Sangeetha S, Biswajit M, Joydeb P, et al. Aeromonas hydrophila OmpW PLGA nanoparticle oral vaccine shows a dose-dependent protective immunity in Rohu (Labeo rohita) Vaccines. 2016;4(2):21. doi: 10.3390/vaccines4020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Samuel CJ. Characterization of poly(d,l-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22:2406–2412. doi: 10.1016/j.vaccine.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 142.Tan Z, Liu W, Liu H, Li C, Zhang Y, Meng X, et al. Oral Helicobacter pylori vaccine-encapsulated acid-resistant HP55/PLGA nanoparticles promote immune protection. Eur J Pharm Biopharm. 2017;111:33–43. doi: 10.1016/j.ejpb.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 143.Munang’andu HM, Evensen Ø. A review of intra- and extracellular antigen delivery systems for virus vaccines of finfish. J Immunol Res. 2015;2015:960859. doi: 10.1155/2015/960859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salvador A, Sandgren KJ, Liang F, Thompson EA, Koup RA, Pedraz JL, et al. Design and evaluation of surface and adjuvant modified PLGA microspheres for uptake by dendritic cells to improve vaccine responses. Int J Pharm. 2015;496(2):371–381. doi: 10.1016/j.ijpharm.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 145.Da Silva CA, Pochard P, Lee CG, Elias JA. Chitin particles are multifaceted immune adjuvants. Am J Respir Crit Care Med. 2010;182(12):1482–1491. doi: 10.1164/rccm.200912-1877OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ghadi A, Mahjoub S, Tabandeh F, Talebnia F. Synthesis and optimization of chitosan nanoparticles: potential applications in nanomedicine and biomedical engineering. Casp J Intern Med. 2014;5(3):156. [PMC free article] [PubMed] [Google Scholar]
- 147.Ponce M, Zuasti E, Reales E, Anguís V, Fernández-Díaz C. Evaluation of an oral DNA nanovaccine against photobacteriosis in Solea senegalensis. Fish Shellfish Immunol. 2021;117:157–168. doi: 10.1016/j.fsi.2021.07.023. [DOI] [PubMed] [Google Scholar]
- 148.Huang X, Ma Y, Wang Y, Niu C, Liu Z, Yao X, et al. Oral probiotic vaccine expressing koi herpesvirus (KHV) ORF81 protein delivered by chitosan-alginate capsules is a promising strategy for mass oral vaccination of carps against KHV infection. J Virol. 2021;95(12):e00415–e421. doi: 10.1128/JVI.00415-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li Y, Zhu Y, Sha T, Chen Z, Yu M, Zhang F, et al. A multi-epitope chitosan nanoparticles vaccine of canine against Echinococcus granulosus. J Biomed Nanotechnol. 2021;17(5):910–920. doi: 10.1166/jbn.2021.3065. [DOI] [PubMed] [Google Scholar]
- 150.Liu X, Sun W, Wu N, Rong N, Kang C, Jian S, et al. Synthesis of Escherichia coli OmpA oral nanoparticles and evaluation of immune functions against the major etiologic agent of cow mastitis. Vaccines. 2021;9(3):304. doi: 10.3390/vaccines9030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Renu S, Han Y, Dhakal S, Lakshmanappa YS, Ghimire S, Feliciano-Ruiz N, et al. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohyd Polym. 2020;243:116434. doi: 10.1016/j.carbpol.2020.116434. [DOI] [PubMed] [Google Scholar]
- 152.Li X, Xing R, Xu C, Liu S, Qin Y, Li K, et al. Immunostimulatory effect of chitosan and quaternary chitosan: a review of potential vaccine adjuvants. Carbohydr Polym. 2021;264:118050. doi: 10.1016/j.carbpol.2021.118050. [DOI] [PubMed] [Google Scholar]
- 153.Abkar M, Fasihi-Ramandi M, Kooshki H, Lotfi AS. Oral immunization of mice with Omp31-loaded N-trimethyl chitosan nanoparticles induces high protection against Brucella melitensis infection. Int J Nanomed. 2017;12:8769. doi: 10.2147/IJN.S149774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dai C, Kang H, Yang W, Sun J, Liu C, Cheng G, et al. O-2′-hydroxypropyltrimethyl ammonium chloride chitosan nanoparticles for the delivery of live Newcastle disease vaccine. Carbohyd Polym. 2015;130:280–289. doi: 10.1016/j.carbpol.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 155.Xu B, Zhang W, Chen Y, Xu Y, Wang B, Zong L. Eudragit® L100-coated mannosylated chitosan nanoparticles for oral protein vaccine delivery. Int J Biol Macromol. 2018;113:534–542. doi: 10.1016/j.ijbiomac.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 156.Shukla A, Mishra V, Bhoop BS, Katare OP. Alginate coated chitosan microparticles mediated oral delivery of diphtheria toxoid (Part A). Systematic optimization, development and characterization. Int J Pharm. 2015;495(1):220–233. doi: 10.1016/j.ijpharm.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 157.Yu X, Wen T, Cao P, Shan L, Li L. Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. J Colloid Interface Sci. 2019;556:258–265. doi: 10.1016/j.jcis.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 158.Jass J, Allison L, Stewart S, Lane M. Ulex europaeus agglutinin-1 binding in hereditary bowel cancer. Pathology. 1993;25(2):114–119. doi: 10.3109/00313029309084782. [DOI] [PubMed] [Google Scholar]
- 159.Foster N, Clark MA, Jepson MA, Hirst BH. Ulex europaeus 1 lectin targets microspheres to mouse Peyer's patch M-cells in vivo. Vaccine. 1998;16(5):536–541. doi: 10.1016/S0264-410X(97)00222-3. [DOI] [PubMed] [Google Scholar]
- 160.Ma T, Wang L, Yang T, Ma G, Wang S. M-cell targeted polymeric lipid nanoparticles containing a toll-like receptor agonist to boost oral immunity. Int J Pharm. 2014;473(1–2):296–303. doi: 10.1016/j.ijpharm.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 161.Gupta PN, Vyas SP. Investigation of lectinized liposomes as M-cell targeted carrier-adjuvant for mucosal immunization. Colloids Surf B. 2011;82(1):118–125. doi: 10.1016/j.colsurfb.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 162.Courtney AN, Nehete PN, Nehete BP, Thapa P, Zhou D, Sastry KJ. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of HIV peptide antigens. Vaccine. 2009;27(25–26):3335–3341. doi: 10.1016/j.vaccine.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lindqvist M, Persson J, Thörn K, Harandi AM. The mucosal adjuvant effect of α-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J Immunol. 2009;182(10):6435–6443. doi: 10.4049/jimmunol.0900136. [DOI] [PubMed] [Google Scholar]
- 164.Davitt CJ, McNeela EA, Longet S, Tobias J, Aversa V, McEntee CP, et al. A novel adjuvanted capsule based strategy for oral vaccination against infectious diarrhoeal pathogens. J Control Release. 2016;233:162–173. doi: 10.1016/j.jconrel.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 165.Longet S, Abautret-Daly A, Davitt CJ, McEntee CP, Aversa V, Rosa M, et al. An oral alpha-galactosylceramide adjuvanted Helicobacter pylori vaccine induces protective IL-1R-and IL-17R-dependent Th1 responses. NPJ Vaccines. 2019;4(1):1–10. doi: 10.1038/s41541-019-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lawson LB, Norton EB, Clements JD. Defending the mucosa: adjuvant and carrier formulations for mucosal immunity. Curr Opin Immunol. 2011;23(3):414–420. doi: 10.1016/j.coi.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 167.Dowling DJ. Recent advances in the discovery and delivery of TLR7/8 agonists as vaccine adjuvants. Immunohorizons. 2018;2(6):185–197. doi: 10.4049/immunohorizons.1700063. [DOI] [PubMed] [Google Scholar]
- 168.Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Nkolola JP, et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540(7632):284–287. doi: 10.1038/nature20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gungor B, Yagci FC, Tincer G, Bayyurt B, Alpdundar E, Yildiz S, et al. CpG ODN nanorings induce IFNα from plasmacytoid dendritic cells and demonstrate potent vaccine adjuvant activity. Sci Transl Med. 2014;6(235):235ra61. doi: 10.1126/scitranslmed.3007909. [DOI] [PubMed] [Google Scholar]
- 170.Carpentier AF. Cancer immunotherapy with CpG-ODN. Med Sci M/S. 2005;21(1):73–77. doi: 10.1051/medsci/200521173. [DOI] [PubMed] [Google Scholar]
- 171.Alignani D, Maletto B, Liscovsky M, Rópolo A, Morón G, Pistoresi-Palencia MC. Orally administered OVA/CpG-ODN induces specific mucosal and systemic immune response in young and aged mice. J Leukoc Biol. 2005;77(6):898–905. doi: 10.1189/jlb.0604330. [DOI] [PubMed] [Google Scholar]
- 172.Singh SM, Alkie TN, Abdelaziz KT, Hodgins DC, Novy A, Nagy E, et al. Characterization of immune responses to an inactivated avian influenza virus vaccine adjuvanted with nanoparticles containing CpG ODN. Viral Immunol. 2016;29(5):269–275. doi: 10.1089/vim.2015.0144. [DOI] [PubMed] [Google Scholar]
- 173.Kwon HC, Kang YJ. Effects of a subunit vaccine (FlaA) and immunostimulant (CpG-ODN 1668) against Vibrio anguillarum in tilapia (Oreochromis niloticus) Aquaculture. 2016;454:125–129. doi: 10.1016/j.aquaculture.2015.12.005. [DOI] [Google Scholar]
- 174.Bai G, Yu H, Guan X, Zeng F, Liu X, Chen B, et al. CpG immunostimulatory oligodeoxynucleotide 1826 as a novel nasal ODN adjuvant enhanced the protective efficacy of the periodontitis gene vaccine in a periodontitis model in SD rats. BMC Oral Health. 2021;21(1):1–12. doi: 10.1186/s12903-021-01763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hjelm BE, Kilbourne J, Herbst-Kralovetz MM. TLR7 and 9 agonists are highly effective mucosal adjuvants for norovirus virus-like particle vaccines. Hum Vaccine Immunother. 2014;10(2):410–416. doi: 10.4161/hv.27147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chadwick S, Kriegel C, Amiji M. Nanotechnology solutions for mucosal immunization. Adv Drug Deliv Rev. 2010;62(4–5):394–407. doi: 10.1016/j.addr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 177.Devriendt B, De Geest BG, Goddeeris BM, Cox E. Crossing the barrier: targeting epithelial receptors for enhanced oral vaccine delivery. J Control Release. 2012;160(3):431–439. doi: 10.1016/j.jconrel.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 178.Sarti F, Perera G, Hintzen F, Kotti K, Karageorgiou V, Kammona O, et al. In vivo evidence of oral vaccination with PLGA nanoparticles containing the immunostimulant monophosphoryl lipid A. Biomaterials. 2011;32(16):4052–4057. doi: 10.1016/j.biomaterials.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 179.Salman HH, Irache JM, Gamazo C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly (anhydride) nanoparticles in oral vaccination. Vaccine. 2009;27(35):4784–4790. doi: 10.1016/j.vaccine.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 180.Mishra N, Tiwari S, Vaidya B, Agrawal GP, Vyas SP. Lectin anchored PLGA nanoparticles for oral mucosal immunization against hepatitis B. J Drug Target. 2011;19(1):67–78. doi: 10.3109/10611861003733946. [DOI] [PubMed] [Google Scholar]
- 181.Oliveira CR, Rezende CM, Silva MR, Pêgo AP, Borges O, Goes AM. A new strategy based on SmRho protein loaded chitosan nanoparticles as a candidate oral vaccine against schistosomiasis. PLoS Negl Trop Dis. 2012;6(11):e1894. doi: 10.1371/journal.pntd.0001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Borges O, Tavares J, de Sousa A, Borchard G, Junginger HE, Cordeiro-da-Silva A. Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles. Eur J Pharm Sci. 2007;32(4–5):278–290. doi: 10.1016/j.ejps.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 183.Taha-Abdelaziz K, Hodgins DC, Alkie TN, Quinteiro-Filho W, Yitbarek A, Astill J, et al. Oral administration of PLGA-encapsulated CpG ODN and Campylobacter jejuni lysate reduces cecal colonization by Campylobacter jejuni in chickens. Vaccine. 2018;36(3):388–394. doi: 10.1016/j.vaccine.2017.11.073. [DOI] [PubMed] [Google Scholar]
- 184.Neutra MR, Pringault E, Kraehenbuhl J-P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14(1):275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 185.Jiang T, Singh B, Li H-S, Kim Y-K, Kang S-K, Nah J-W, et al. Targeted oral delivery of BmpB vaccine using porous PLGA microparticles coated with M cell homing peptide-coupled chitosan. Biomaterials. 2014;35(7):2365–2373. doi: 10.1016/j.biomaterials.2013.11.073. [DOI] [PubMed] [Google Scholar]
- 186.Yang Y, Teng Z, Lu Y, Luo X, Mu S, Ru J, et al. Enhanced immunogenicity of foot and mouth disease DNA vaccine delivered by PLGA nanoparticles combined with cytokine adjuvants. Res Vet Sci. 2021;136:89–96. doi: 10.1016/j.rvsc.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 187.Akhtar M, Chowdhury MI, Bhuiyan TR, Kaim J, Ahmed T, Rafique TA, et al. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled Phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine. 2019;37(37):5645–5656. doi: 10.1016/j.vaccine.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qadri F, Akhtar M, Bhuiyan TR, Chowdhury MI, Ahmed T, Rafique TA, et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2020;20(2):208–219. doi: 10.1016/S1473-3099(19)30571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McGonigle P, Ruggeri B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol. 2014;87(1):162–171. doi: 10.1016/j.bcp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 190.Jones KS. Biomaterials as vaccine adjuvants. Biotechnol Prog. 2008;24(4):807–814. doi: 10.1002/btpr.10. [DOI] [PubMed] [Google Scholar]
- 191.Mohan T, Verma P, Rao DN. Novel adjuvants and delivery vehicles for vaccines development: a road ahead. Indian J Med Res. 2013;138(5):779. [PMC free article] [PubMed] [Google Scholar]
- 192.Cunningham AF, Khan M, Ball J, Toellner KM, Serre K, Mohr E, et al. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur J Immunol. 2004;34(11):2986–2995. doi: 10.1002/eji.200425403. [DOI] [PubMed] [Google Scholar]
- 193.Cummings LA, Barrett SLR, Wilkerson WD, Fellnerova I, Cookson BT. FliC-specific CD4+ T cell responses are restricted by bacterial regulation of antigen expression. J Immunol. 2005;174(12):7929–7938. doi: 10.4049/jimmunol.174.12.7929. [DOI] [PubMed] [Google Scholar]
- 194.Yuki Y, Mejima M, Kurokawa S, Hiroiwa T, Takahashi Y, Tokuhara D, et al. Induction of toxin-specific neutralizing immunity by molecularly uniform rice-based oral cholera toxin B subunit vaccine without plant-associated sugar modification. Plant Biotechnol J. 2013;11(7):799–808. doi: 10.1111/pbi.12071. [DOI] [PubMed] [Google Scholar]
- 195.Martin D, Ruijne N, McCallum L, O’hallahan J, Oster P. The VR2 epitope on the PorA P1. 7–2, 4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin Vaccine Immunol. 2006;13(4):486–491. doi: 10.1128/CVI.13.4.486-491.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.El-Kamary SS, Cohen MB, Bourgeois AL, Van De Verg L, Bauers N, Reymann M, et al. Safety and immunogenicity of a single oral dose of recombinant double mutant heat-labile toxin derived from enterotoxigenic Escherichia coli. Clin Vaccine Immunol. 2013;20(11):1764–1770. doi: 10.1128/CVI.00464-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ma S, Qiao X, Xu Y, Wang L, Zhou H, Jiang Y, et al. Screening and identification of a chicken dendritic cell binding peptide by using a phage display library. Front Immunol. 2019;10:1853. doi: 10.3389/fimmu.2019.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.An S-B, Oh S-H, Lee J-Y, Choi K-H, Lee C-K, Choi Y-J, et al. Identification of a porcine TLR2-targeting peptide ligands using a cell-based phage display combined with high-throughput sequencing. 2020. 10.21203/rs.3.rs-124838/v1.
- 199.Luo Q, Vickers TJ, Fleckenstein JM. Immunogenicity and protective efficacy against enterotoxigenic Escherichia coli colonization following intradermal, sublingual, or oral vaccination with EtpA adhesin. Clin Vaccine Immunol. 2016;23(7):628–637. doi: 10.1128/CVI.00248-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ottsjö LS, Flach C-F, Clements J, Holmgren J, Raghavan S. A double mutant heat-labile toxin from Escherichia coli, LT (R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect Immun. 2013;81(5):1532–1540. doi: 10.1128/IAI.01407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhong Y, Chen J, Liu Y, Zhang Y, Tang C, Wang X, et al. Oral immunization of BALB/c mice with recombinant Helicobacter pylori antigens and double mutant heat-labile toxin (dmLT) induces prophylactic protective immunity against H. pylori infection. Microb Pathog. 2020;145:104229. doi: 10.1016/j.micpath.2020.104229. [DOI] [PubMed] [Google Scholar]
- 202.Mishra N, Smyth JA. Oral vaccination of broiler chickens against necrotic enteritis using a non-virulent NetB positive strain of Clostridium perfringens type A. Vaccine. 2017;35(49):6858–6865. doi: 10.1016/j.vaccine.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 203.Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci Transl Med. 2013;5(213):213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Christophe M, Kuczkowska K, Langella P, Eijsink VG, Mathiesen G, Chatel J-M. Surface display of an anti-DEC-205 single chain Fv fragment in Lactobacillus plantarum increases internalization and plasmid transfer to dendritic cells in vitro and in vivo. Microb Cell Fact. 2015;14(1):95. doi: 10.1186/s12934-015-0290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dubey S, Avadhani K, Mutalik S, Sivadasan SM, Maiti B, Paul J, et al. Aeromonas hydrophila OmpW PLGA nanoparticle oral vaccine shows a dose-dependent protective immunity in rohu (Labeo rohita) Vaccines. 2016;4(2):21. doi: 10.3390/vaccines4020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu M, Thijssen S, van Nostrum CF, Hennink WE, Garssen J, Willemsen LE. Inhibition of cow’s milk allergy development in mice by oral delivery of β‐lactoglobulin‐derived peptides loaded PLGA nanoparticles is associated with systemic whey‐specific immune silencing. Clin Exp Allergy. 2021;52(1):137–48. [DOI] [PMC free article] [PubMed]
- 207.Saint-Lu N, Tourdot S, Razafindratsita A, Mascarell L, Berjont N, Chabre H, et al. Targeting the allergen to oral dendritic cells with mucoadhesive chitosan particles enhances tolerance induction. Allergy. 2009;64(7):1003–1013. doi: 10.1111/j.1398-9995.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 208.Zhang F, Hao C, Zhang S, Li A, Zhang Q, Wu W, et al. Oral immunization with recombinant enterovirus 71 VP1 formulated with chitosan protects mice against lethal challenge. Virol J. 2014;11(1):80. doi: 10.1186/1743-422X-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dubey S, Avadhani K, Mutalik S, Sivadasan SM, Maiti B, Girisha SK, et al. Edwardsiella tarda OmpA encapsulated in chitosan nanoparticles shows superior protection over inactivated whole cell vaccine in orally vaccinated fringed-lipped peninsula carp (Labeo fimbriatus) Vaccines. 2016;4(4):40. doi: 10.3390/vaccines4040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Malik B, Goyal AK, Markandeywar T, Rath G, Zakir F, Vyas SP. Microfold-cell targeted surface engineered polymeric nanoparticles for oral immunization. J Drug Target. 2012;20(1):76–84. doi: 10.3109/1061186X.2011.611516. [DOI] [PubMed] [Google Scholar]
- 211.Onuigbo E, Iseghohimhen J, Chah K, Gyang M, Attama A. Chitosan/alginate microparticles for the oral delivery of fowl typhoid vaccine: Innate and acquired immunity. Vaccine. 2018;36(33):4973–4978. doi: 10.1016/j.vaccine.2018.05.087. [DOI] [PubMed] [Google Scholar]
- 212.Zhao K, Zhang Y, Zhang X, Shi C, Wang X, Wang X, et al. Chitosan-coated poly (lactic-co-glycolic) acid nanoparticles as an efficient delivery system for Newcastle disease virus DNA vaccine. Int J Nanomed. 2014;9:4609. doi: 10.2147/IJN.S70633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Renu S, Markazi AD, Dhakal S, Lakshmanappa YS, Shanmugasundaram R, Selvaraj RK, et al. Oral deliverable mucoadhesive chitosan-salmonella subunit nanovaccine for layer chickens. Int J Nanomed. 2020;15:761. doi: 10.2147/IJN.S238445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Soares E, Jesus S, Borges O. Oral hepatitis B vaccine: chitosan or glucan based delivery systems for efficient HBsAg immunization following subcutaneous priming. Int J Pharm. 2018;535(1–2):261–271. doi: 10.1016/j.ijpharm.2017.11.009. [DOI] [PubMed] [Google Scholar]