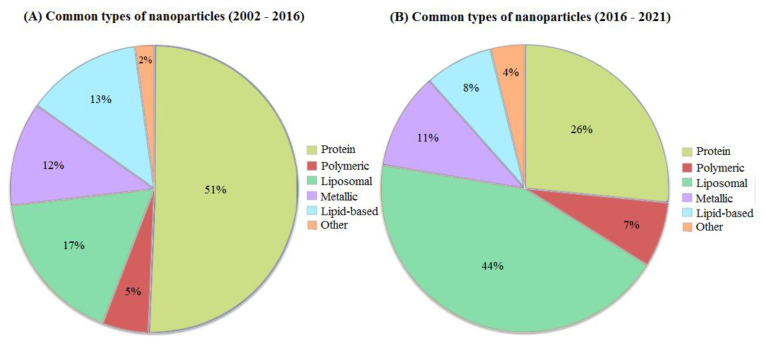
Figure 2.
Common types of nanoparticles. This figure contains information about the types of nanoparticles used in clinical trials. (A) Pie chart A represents the types of nanoparticles in clinical trials from 2002 to 2016. The group other consists of carbon-based, silica-based nanoparticles and nanostructured formulations of hormones. In the 2002–2016 period, the most abundant type of nanoparticles in clinical trials was protein (51%). Liposomal formulations were the second most common but still relatively low (17%). Lipid-based nanoparticles could be encountered in 13% of all clinical trials in that period. Both metallic and polymeric formulations appeared to be scarce (12 and 5% respectively). (B) This part of the figure represents types of nanoparticles in trials from 2016 to 2021. The group other consists of quantum dots, micellar nanoparticles and exosomes. In comparison to 2002–2016, protein nanoparticles demonstrated a downfall (from 51% to 26%) which can be explained by an increase in liposomal drugs (from 17% to 44%). Lipid-based formulations also faced a slight decrease to 8%, while metallic and polymeric drug percentages stayed almost the same (11% and 7%, respectively).