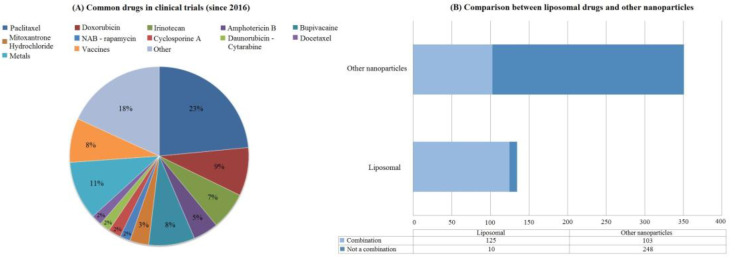
Figure 3.
Common drugs and liposomal drugs application. The categories in (A) were formed based on the most common studied drugs. Mostly, each drug was mentioned individually except for the groups metals, vaccine and other. The first two groups were formed based on the heterogeneity of the drugs they comprise. The vaccine group most often consisted of mRNA vaccines, while the Metals group was dominated by various iron preparations. Others consisted of drugs that occurred no more than two times in the entire excerpt. Paclitaxel consisted of various nanoparticle types that were loaded with paclitaxel. Among them, NAB-paclitaxel was occurring the most often (57 trials out of 62 trials for paclitaxel in total). Chemotherapy drugs in general were the most common drugs in the dataset (paclitaxel—23%, doxorubicin—9%, irinotecan—7%). Different vaccines and metals were also often mentioned in clinical trials (8% and 11% respectively). Local anesthetic bupivacaine was also frequently incorporated in nanoparticle systems (8%). Obtained results could be explained by various properties that nanoparticles can have as drug carriers (for instance, site-specific drug delivery). (B) shows the division of clinical trials that studied liposomal drugs either individually or in combination with other regular drugs. Based on (B), liposomal drugs were primarily used in combination with other drugs (125 clinical trials with combinations out of a total of 135 liposomal trials). Such a phenomenon can be explained by a small amount of liposomal clinical trials in comparison with all the other types of nanoparticles. The second possible reason can be due to specific diseases (for instance, neoplasms) that require a combination of therapeutic agents and prevail in our dataset. mRNA—messenger ribonucleic acid, NAB—nanoparticle albumin-bound.