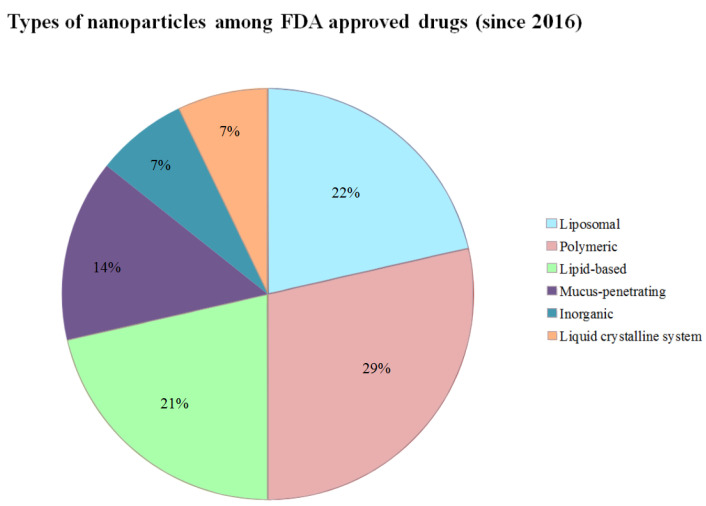
Figure 5.
Common types of nanoparticles among FDA-approved drugs (since 2016). This figure depicts the types of nanoparticles that were used in FDA-approved medicine (since 2016). The most common were polymeric drugs (29%). The second most common were liposomal drugs (22%). However, lipid-based formulations appeared to be very close to liposomal drugs among FDA-approved medicine since 2016 (21%). Mucus-penetrating nanoparticles (14%) were applied primarily in ocular surgery to reduce post-operative inflammation. Inorganic nanoparticles and liquid crystalline system appeared only once in the table; therefore, being equal to 7% for each. This figure illustrates that polymeric, lipid-based and liposomal nanoparticles can show better results in clinical trials reaching an approval stage. On the other side, the presence of such rare and relatively new categories of nanoparticles as mucus-penetrating and liquid crystalline systems emphasizes the need for further research and development of drug-carrier systems.