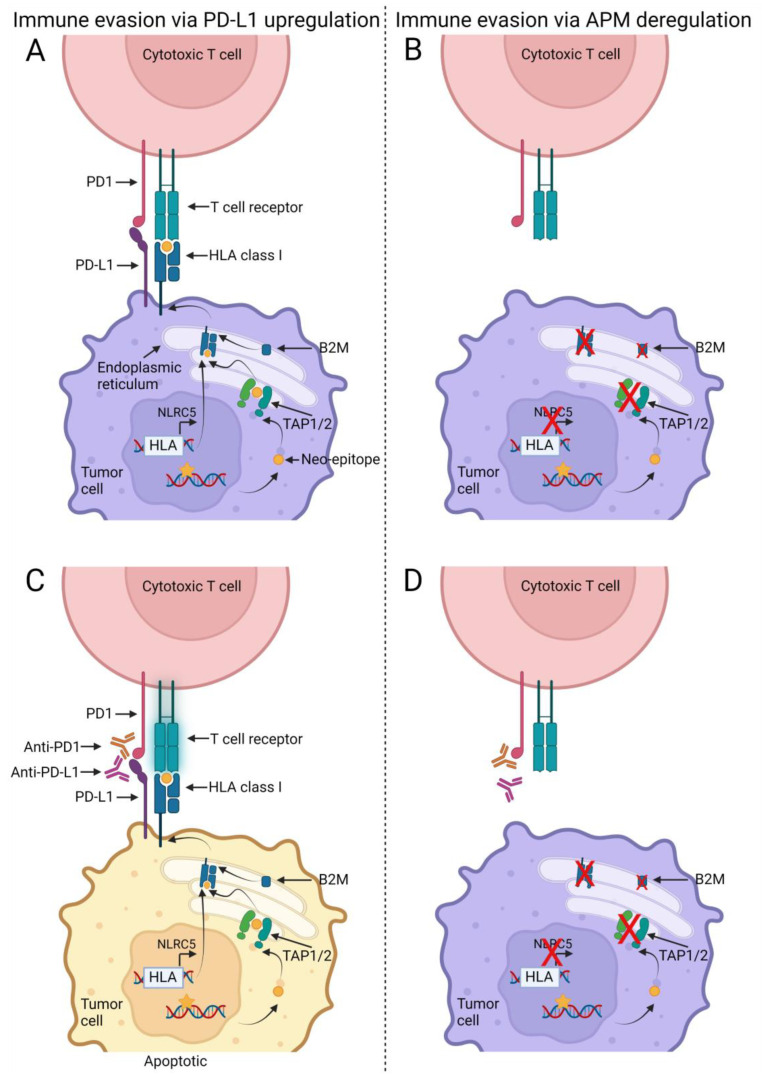
Figure 1.
Model for non-responders and responders in relation to immune checkpoint inhibitors (ICI) and antigen presentation machinery (APM). DNA mutations (yellow star) generated by replicative errors during tumor cell division may result in altered protein structures. These proteins are degraded into smaller fragments (neo-epitopes) (yellow circle) that are imported into the endoplasmic reticulum and loaded onto the human leukocyte antigen (HLA) class I receptors by the transporter associated with antigen processing (TAP) 1 and TAP2. Prior to the loading the HLA class I molecule has been assembled with its essential subunit, beta-2 microglobulin (B2M). The assembled HLA class I receptor is transported to the cell surface where the T cell receptor on the cytotoxic T cells recognize the neo-epitope as foreign and in turn induce apoptosis in the tumor cell. The tumor cell can up-regulate the programmed death 1 ligand 1 (PD-L1) receptor and thereby inhibit the activity of the T cell (A). This interaction between PD-L1 and programmed death 1 (PD-1) can be inhibited by ICI therapy with anti-PD-L1 or anti-PD1 drugs and thereby re-activate the T cells (B). However, some tumors may evade the T cell mediated attack via deregulations of the APM proteins, such as B2M or HLA class I loss-of-function mutations (C). In theory, ICI therapy, reactivating the cytotoxic T cells, will not have any effect on the tumor cells as the T cells cannot identify the tumor cells (D). Created with BioRender.com, acceded on 18 November 2022.