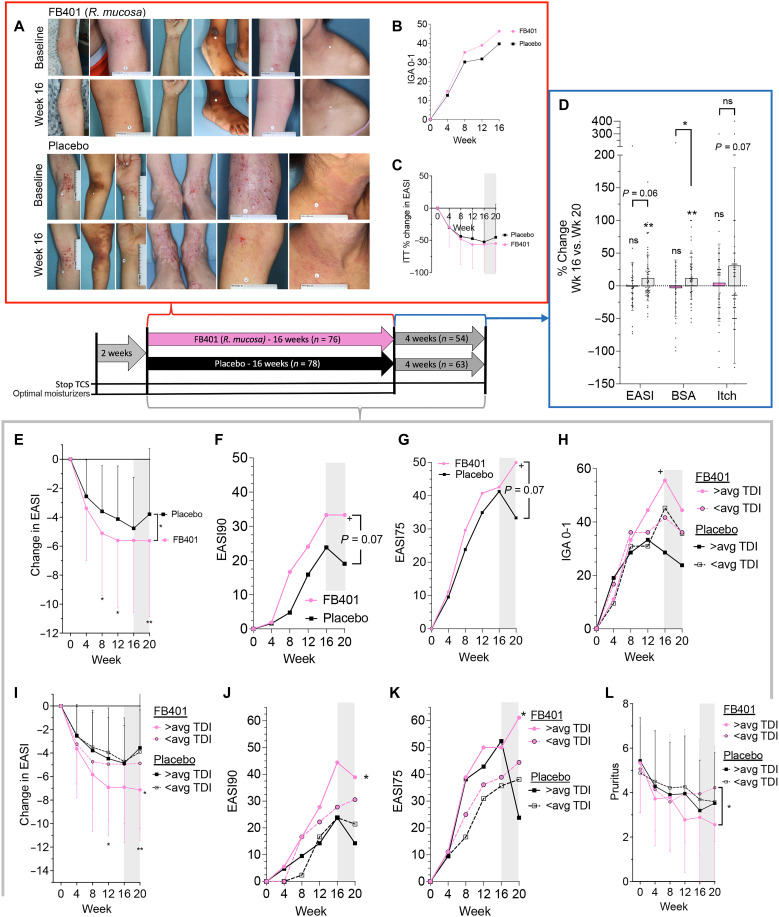
Fig. 6. Clinical results from R. mucosa treatment were influenced by TDI exposure.
Patients were treated with topical R. mucosa (FB-401) or placebo (sucrose in water) thrice weekly for 16 weeks before a 4-week washout. Two weeks before treatment, topical corticosteroids (TCS) were stopped and a minimally dysbiotic moisturizer regiment was initiated. (A) Example images from participants. (B) ITT analysis for those achieving an IGA of 0 and 1 (C) and percent change in EASI during active treatment. (D) For those completing the washout phase, percent change between week 16 and week 20 as a percentage of enrollment values is shown for EASI, BSA, and pruritis NRS (itch); each dot represents one participant. (E to L) For those completing the entire 20-week trial without topical steroids, raw EASI change (E), the proportion achieving 90% improvement in EASI (EASI90; F), and EASI75 (G). (H to L) Post hoc stratification of the completer population results by study sites’ historical TDI exposure as above the mean (>avg) or below for IGA0-1 (H), raw EASI (I), EASI90 (J), EASI75 (K), and pruritus (L); significance indicated for FB-401 in above or below average TDI zip codes against matched control unless indicated. Data are shown as proportion (B, F to H, J, and K) and means + SD (C, D, I, and L). ns, not significant, +P < 0.1; *P < 0.05; **P < 0.01 as determined by ANOVA (C and D), rANOVA (E, I, and L), or chi-square (B, F to H, J, and K).