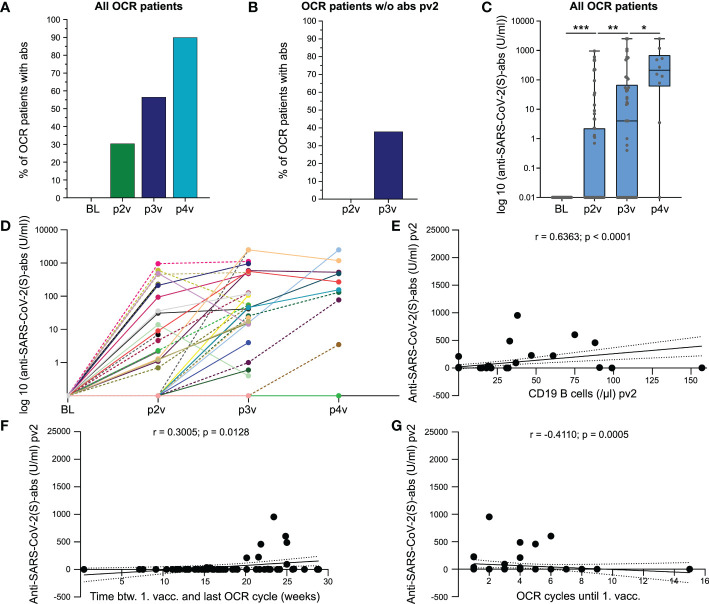
Figure 2.
COVID-19 booster vaccinations can increase humoral immune response in MS patients treated with ocrelizumab. (A) Percentage of OCR-pwMS with positive anti-SARS-CoV-2(S)-antibodies post second, third, and fourth vaccination; (B) Seroconversion after the third vaccine shot in previously antibody negative OCR-pwMS; (C) Anti-SARS-CoV-2(S)-antibody titers post second, third, and fourth vaccination. Values < 0.4 U/ml are depicted on the x-axis; (D) Individual anti-SARS-CoV-2(S)-antibody titers at BL, pv2, pv3 and pv4. Connection lines between the time points serve as orientation and do not reflect the anti-SARS-CoV-2(S)-antibody level between two time points; Correlation of anti-SARS-CoV-2(S)-antibodies with CD19 B cell count pv2 (E), time between first vaccination and last OCR cycle (F), and number of previous OCR cycles (G). Correlation analyses were performed with the Spearman correlation coefficient. The area in-between the dotted lines shows the 95% confidence interval. Abs, antibodies; BL, baseline; btw, between; COVID-19, coronavirus disease 2019; OCR, ocrelizumab; pv2/3/4, post second/third/fourth vaccination; S, spike; SARS-CoV-2, severe acute respiratory syndrome; w/o, without; vacc., vaccination. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.