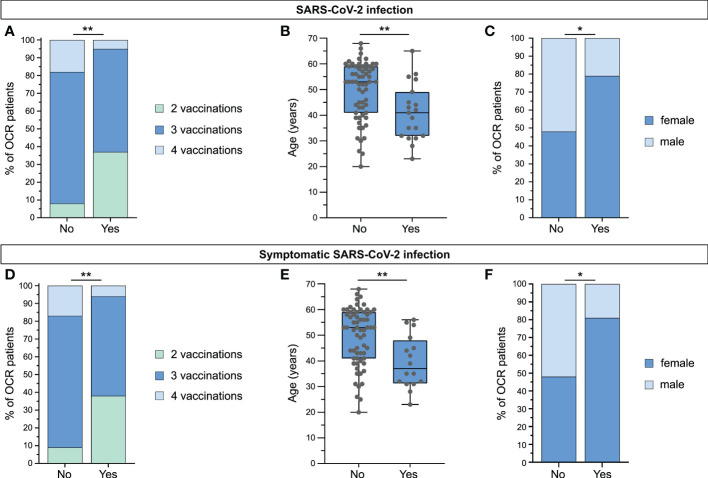
Figure 3.
Vaccine-based clinical protection of MS patients receiving ocrelizumab. (A) Differences in vaccine doses between OCR-pwMS infected with SARS-CoV-2 and non-infected patients; (B) Comparison of age between SARS-CoV-2-infected OCR-pwMS and non-infected patients. (C) Differences in sex between non-infected and infected OCR-pwMS; (D) Comparison of vaccine doses between OCR-pwMS with and without symptomatic SARS-CoV-2 infection; (E) Differences in age between OCR-pwMS with and without symptomatic SARS-CoV-2 infection; (F) Differences in sex between OCR-pwMS with and without symptomatic SARS-CoV-2 infection. *p ≤ 0.05, **p ≤ 0.01. OCR, ocrelizumab; SARS-CoV-2, severe acute respiratory syndrome.