Figure 6. Epsins bind to ABCG1 and facilitate the internalization and degradation of ABCG1 via lysosomes.
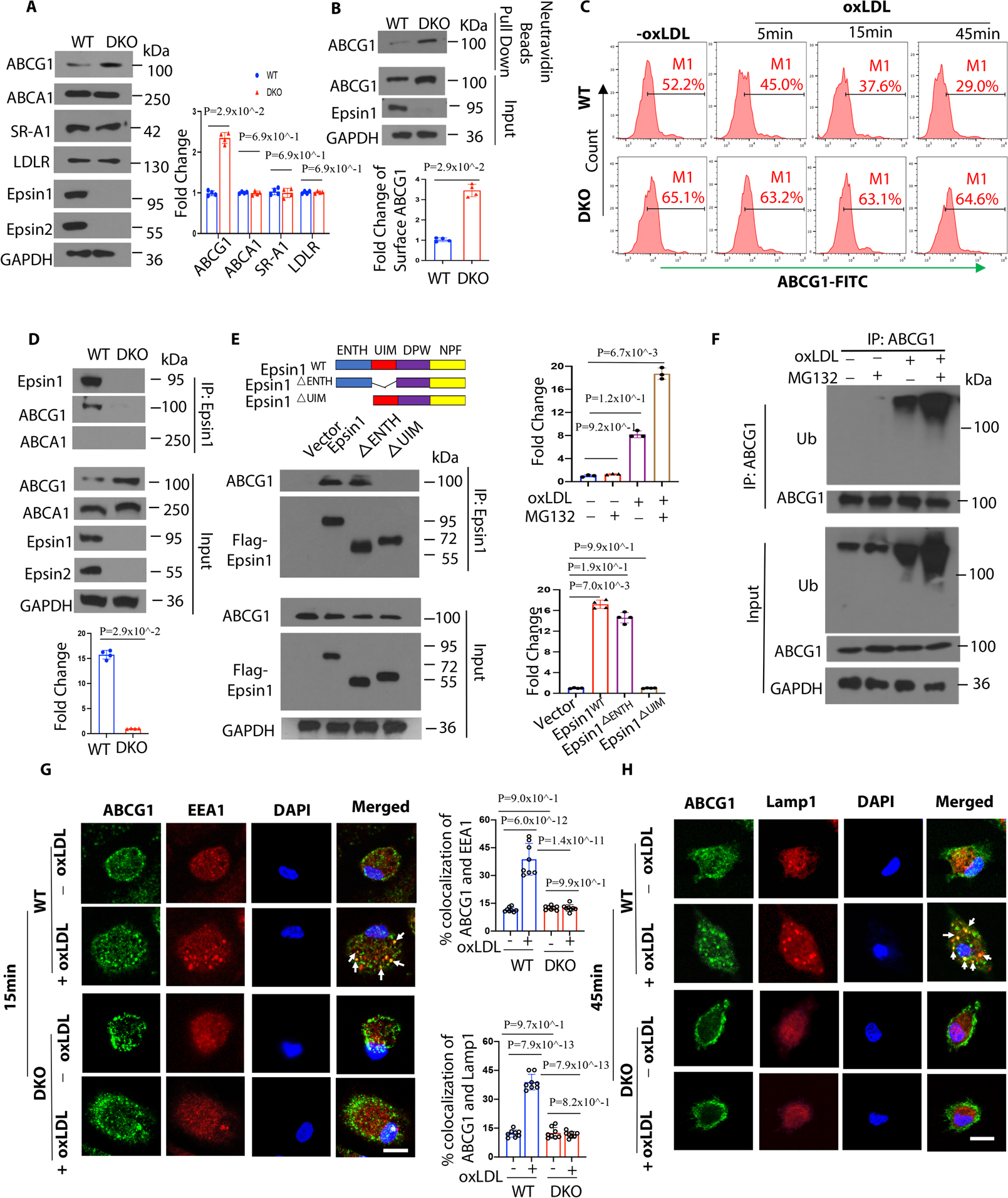
(A) Peritoneal macrophages from WT/ApoE−/− and LysM-DKO/ApoE−/− mice on normal diet (ND) were lysed for WB analysis (n=4). (B) Peritoneal macrophages from WT/ApoE−/− and LysM-DKO/ApoE−/− mice on ND were pretreated with a liver X receptor (LXR) activator and followed by a cell surface biotinylation assay to evaluate the cell surface ABCG1 levels (n=4). (C) LXR agonist activated WT and LysM-DKO peritoneal macrophages were incubated in lipid-deficient medium for 24h and treated with or without 100μg/mL oxLDL for 5, 15 and 45 min followed by staining with anti-ABCG1 and analyzed by flow cytometry. (D) LXR agonist pretreated peritoneal macrophages isolated from WT and LysM-DKO mice on ND were treated with 100μg/mL oxLDL for 1h followed by IP and WB for Epsin1 and ABCG1 or ABCA1 (n=4). (E) ABCG1 plasmids and full length (FLAG-Epsin1WT) or domain-deletion constructs (FLAG-Epsin1ΔENTH or FLAG-Epsin1ΔUIM) in the pcDNA3 vector were transfected into HEK 293T cells for 24 h in the presence of LXR agonist. Cells were then treated with 100μg/mL oxLDL for 1h, followed by IP and WB analysis using antibodies against FLAG tags and ABCG1 (n=4). (F) Peritoneal macrophages isolated from WT mice on ND were cultured in serum-free medium for 24h followed by treatment with 5 μM MG132 for 3h. Cells were then treated with 100μg/mL oxLDL for 1h followed by IP and WB for ubiquitin and ABCG1. (G-H) WT and LysM-DKO peritoneal macrophages were incubated in lipid-deficient medium for 24h followed by incubation with or without 100μg/mL oxLDL for 15min (G) or 45min (H) at 370C. Macrophages were stained with ABCG1 (green), early endosome marker EEA1 (red) or lysosome marker Lamp1 (red) and DAPI (blue), then assessed by confocal microscopy. White arrows indicate the endocytic vesicles, scale bar=5μm, n=8/group. Data from A-H are presented as mean ± SD. Unpaired non-parametric Mann-Whitney U test was conducted in A, B and D; Kruskal-Wallis test followed by Dunn’s post hoc multiple comparisons test was conducted in C, E and F. Two-way ANOVA followed by Sidak post hoc multiple comparisons test was conducted in G and H.
