Figure 1:
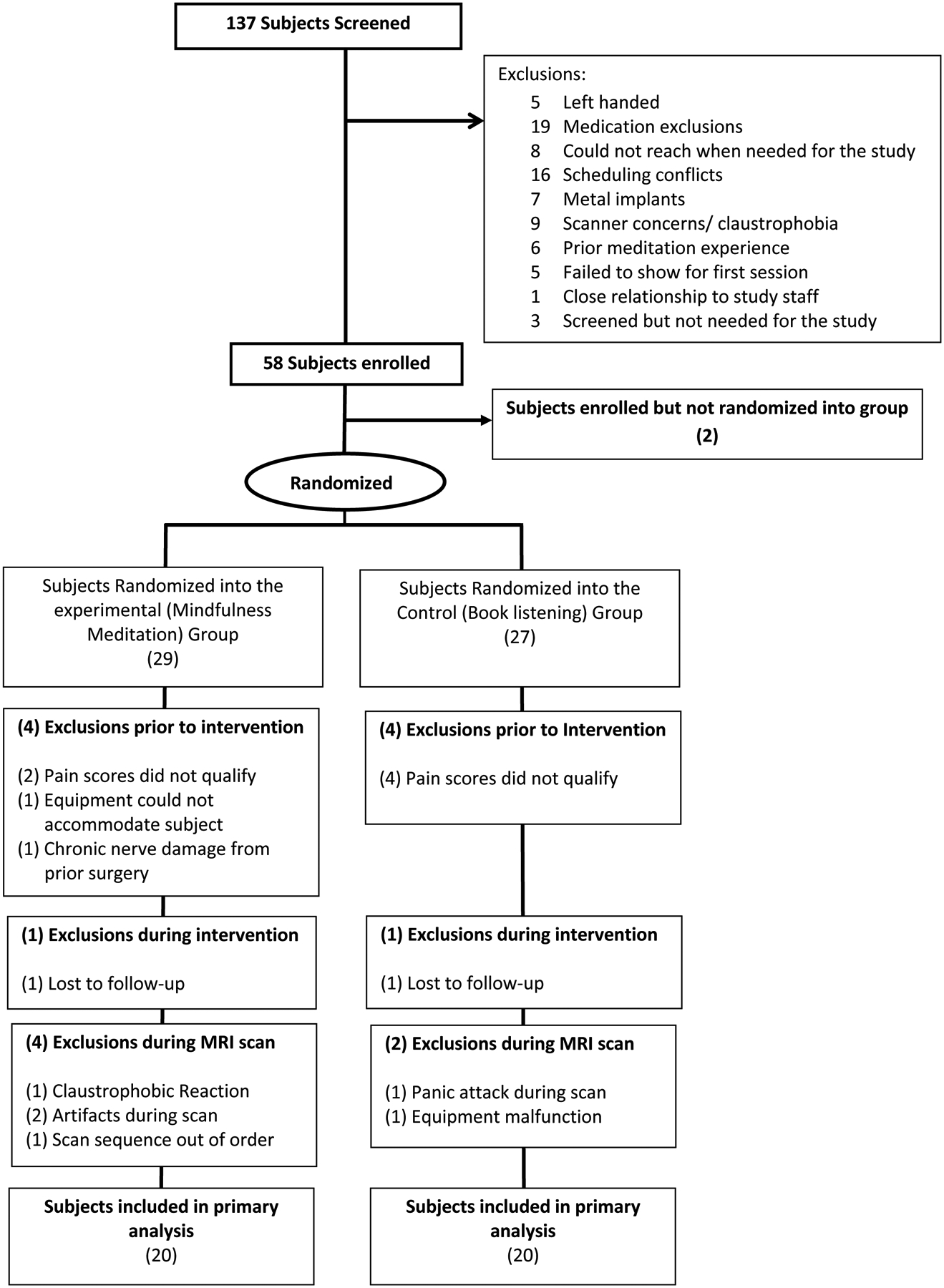
Consolidated Standards of Reporting Trials (CONSORT) flow diagram: At screening, 79 participants were excluded for not meeting inclusion-exclusion criteria. Fifty-eight participants were enrolled into the study. After session 1, participants were dropped for low pain sensitivity (n=6), equipment not fitting (n=1), and chronic pain (n=1). After randomization, a subject from each group was dismissed for “no-shows”. In the MRI session, a total of four participants from the mindfulness group and 2 from the control group were dismissed for several reasons. Two participants completed their respective fMRI scans but were removed from the final analysis (and replaced at random) due to a procedural error and an exhibited fMRI related artifact, respectively. The targeted sample size of forty participants were included in the study.
