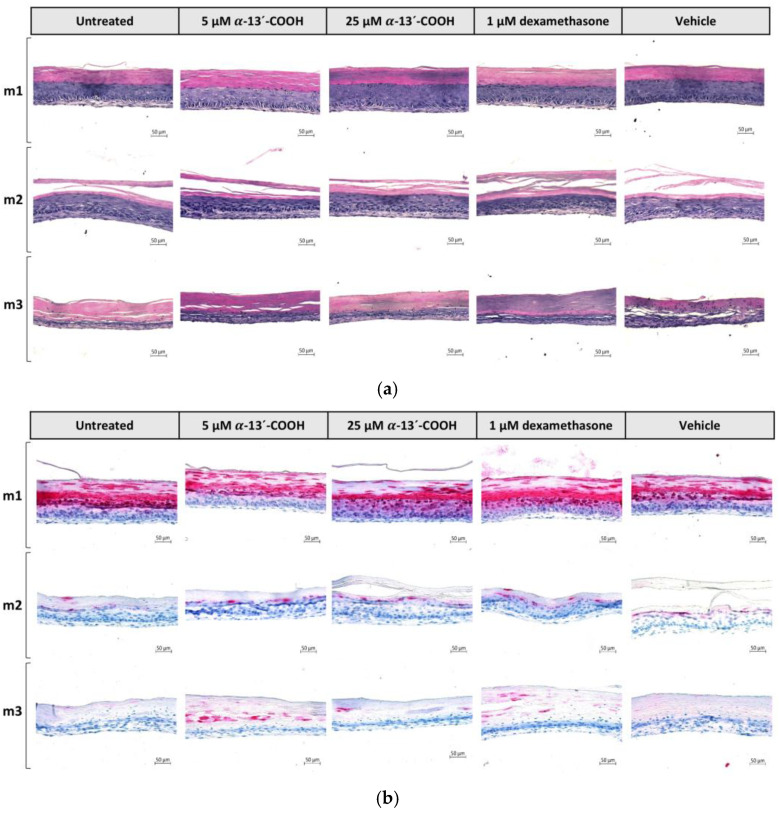
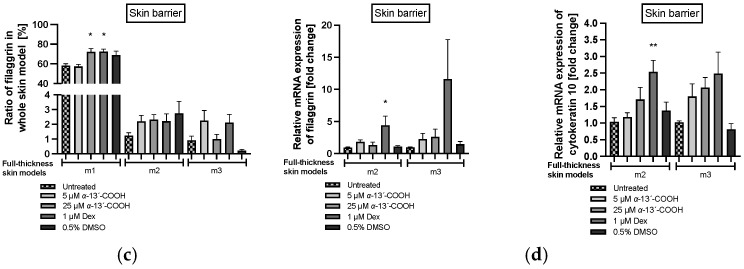
Figure 7.
(a,b) Histological, (c) quantitative evaluation, and (d) gene expression profile of filaggrin in healthy and atopic dermatitis full-thickness skin models after 12 days of cultivation to air surface (airlift). Healthy skin models were cultivated under normal medium conditions (m1). Atopic dermatitis skin models were stimulated with TH2 cytokines (50 ng/mL IL-4, 50 ng/mL IL-13 and 25 ng/mL IL-31) at days 0, 2, 5, 7 and 9 (m2, m3). Atopic dermatitis skin model m3 was additionally stimulated with 10 µM histamine at day 9. Skin models m1, m2 and m3 were pre-incubated with either 5 µM α-13’-COOH, 25 µM α-13’-COOH, 1 µM dexamethasone (Dex; positive control), or 0.5% DMSO (vehicle control) at days 7 and 9, or were left untreated as disease control (untreated). (a) Skin models for evaluation of skin morphology were stained with haematoxylin-eosin and (b) for evaluation of skin barrier effects with filaggrin. Scale bar: 50 µm. (c) Ratio of filaggrin expression in whole skin models is given as the area of filaggrin in whole skin models in relation to the area of the whole skin model in [%] using ImageJ software (Scale bar: 100 µm). (d) Transcript levels are given as relative mRNA expression referred to the untreated disease control [fold change]. (c,d) All data are presented as mean ± SEM. Two independent experiments were performed as replicates. Histological analyses were conducted on three skin models from two independent experiments with four images per skin model. Asterisks [*] indicate significant deviations from the untreated disease control (* p < 0.05 and ** p < 0.01).