Figure 3. Vitamin B3 cycles between host and gut microbiome.
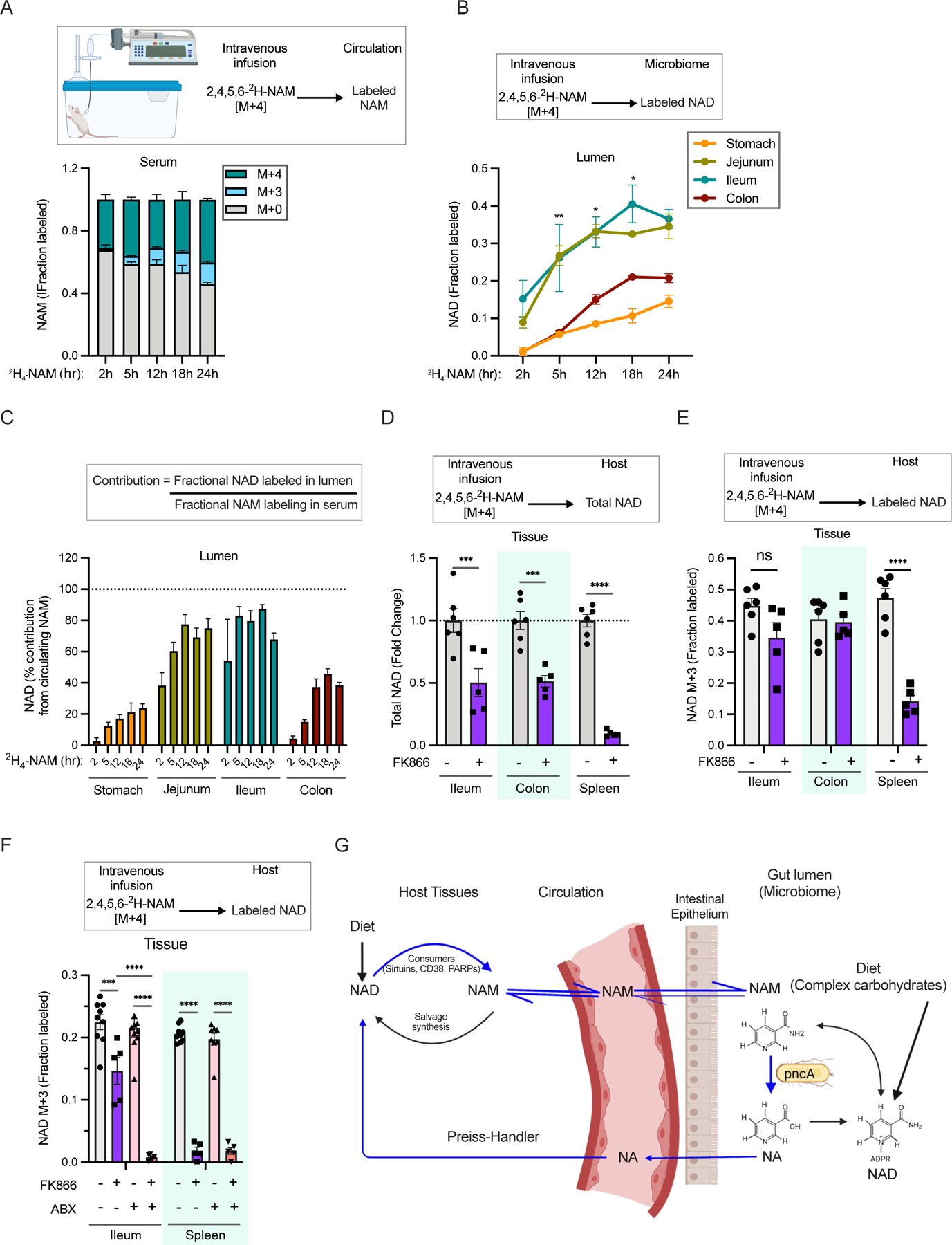
A. Fraction labeling of nicotinamide in the serum of mice infused with 4mM [2,4,5,6-2H]-NAM for different time points.
B. Fraction labeled NAD in the luminal content of mice infused as in A. (n=2–4 per group; Tukey’s multiple comparison test of fractional labeling in the ileum lumen and colon lumen following two-way ANOVA, * = p < 0.05, ** = p < 0.01).
C. Percent contribution of circulating NAM to NAD synthesis in different parts of the gastrointestinal tract estimated from mice infused as in A.
D. Relative levels of total NAD in mice intraperitoneally injected with vehicle or FK866 and infused with [2,4,5,6-2H]-NAM for 23h. (n=5–6 mice per treatment group; Sidak’s multiple comparison test following two-way ANOVA, *** = p < 0.001, **** = p < 0.0001).
E. Fraction labeled NAD in mice treated as in D. (n=5–6 mice per treatment group; Sidak’s multiple comparison test following two-way ANOVA, **** = p < 0.0001). Data for spleen in D and E was previously reported in 40.
F. Fraction labeled NAD in tissues collected from control and antibiotics (ABX) treated mice intraperitoneally injected with vehicle or FK866 and infused with [2,4,5,6-2H]-NAM for 5h. (n=5–10 mice per treatment group; Tukey’s multiple comparison test following two-way ANOVA, *** = p < 0.001, **** = p < 0.0001).
G. Model showing a vitamin B3 cycle between host and microbes. Host-derived NAM in the circulation enters the gut lumen and is deamidated to NA by the microbiome. In turn, host tissues use microbiome-produced NA for synthesis of NAD, which is turned over to release NAM.
