Figure 7.
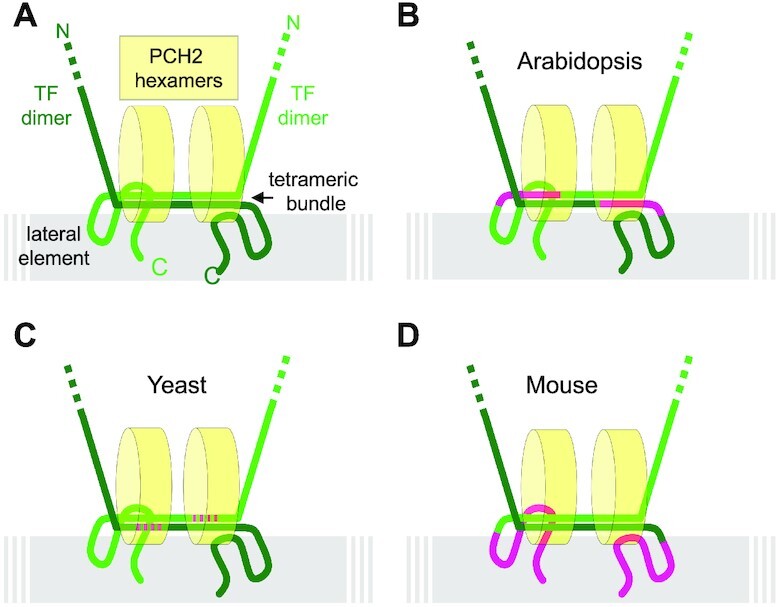
Model for a conserved recruitment mechanism of PCH2 to the SC. (A) Hypothetical generic model of PCH2 binding to the transversal filament that combines information from different organisms and assumes the formation of a similar sized tetrahelical bundle formed by the antiparallel oriented C-terminal ends of two transverse filament dimers, as published for human SYCP1 (57), also occurs in Arabidopsis, mouse and yeast. Two hexameric PCH2 wheels might bind through probably their N-termini to the region of the tetrahelical bundle parallel to the chromosome axis. Alternatively, one hexameric PCH2 wheel might lay flat on top of the tetrahelical bundle. (B–D) The localization of PCH2 at the SC is possibly conserved between diverse organisms although the domains in the transverse filament proteins making the main contact with PCH2 have slightly shifted in evolution. The domains found to be relevant for correct PCH2 binding are indicated in magenta for Arabidopsis (this study), mouse (this study) and Yeast (37).
