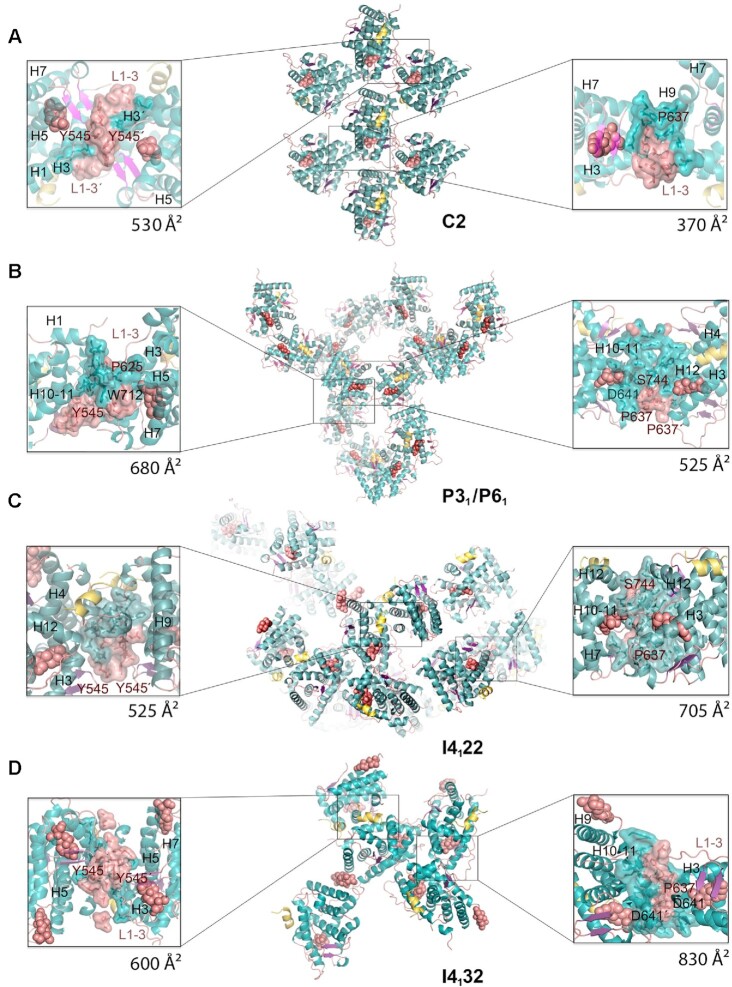
Figure 2.
New crystal structures of GR-LBD·DEX reveal a variety of quaternary assemblies. For all structures, the overall crystal packing is shown in the central panels. Monomers are depicted as cartoons with helices colored blue and loops colored salmon. DEX molecules are represented as salmon spheres and SHP peptides as yellow ribbons. Details of intermonomer interfaces are given in the lateral panels, in which the side chains of interacting residues are shown as sticks. (A) C2 crystals. Note that major contacts are centered on L1–3 with stacked Tyr545 phenol rings from two neighboring molecules. A monoclinic structure of ancGR2-LBD bound to another synthetic GC, triamcinolone acetonide, and complexed to a shorter SHP peptide had been previously reported (5UFS; (38)). Interestingly, 5UFS features a Tyr545-centered parallel dimer almost identical to the topologically equivalent arrangement in our current C2 crystals. (B) Related P31 and P61 crystals. Tyr545 engages in heterologous contacts with a neighboring molecule in these crystals (see the position of the Trp712’ side chain). Structures of ancGR2-LBD bound to either DEX or a different GC (mometasone furoate) and complexed to a TIF-2 peptide had been previously reported in a similar hexagonal crystal form (3GN8 and 4E2J; a and b axes are ∼5% longer in our crystals, while the c axis is ∼4% shorter). These relatively small differences in the cell constants compared to the current P61 structure result in a markedly different small intermonomer interface, however, which is asymmetric in 3GN8/4E2J. (C, D) Common packing of I4122 and I4132 crystals. Note that the phenol rings of two Tyr545 residues stack as in the C2 crystals, although the two interacting monomers are fully differently oriented relative to each other. Note also that the largest interface in these crystals features abutting Asp641 side chains from three monomers organized around a local (I4122) or exact 3-fold axis (I4132; right side panel in D).